Stabilized Carbon Radical-Mediated Assembly of Arylthianthrenium Salts, Alkenes and Amino Acid/Peptide Derivatives
- PMID: 39573977
- PMCID: PMC11727398
- DOI: 10.1002/advs.202411579
Stabilized Carbon Radical-Mediated Assembly of Arylthianthrenium Salts, Alkenes and Amino Acid/Peptide Derivatives
Abstract
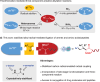
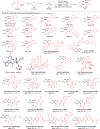
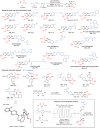
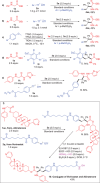
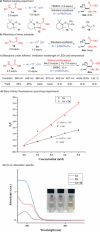
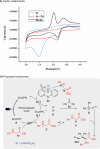
Efficiently assembling amino acids and peptides with bioactive molecules facilitates the modular and streamlined synthesis of a diverse library of peptide-related compounds. Particularly notable is their application in pharmaceutical development, leveraging site-selective late-stage functionalization. Here, a visible light-induced three-component reaction involving arylthianthrenium salts, amino acid/peptide derivatives, and alkenes are introduced. This approach utilizes captodatively-stabilized carbon radicals to enable radical-radical C─C coupling, effectively constructing complex bioactive molecules. This method offers a promising alternative route for modular synthesis of peptide-derived bio-relevant compounds.
Keywords: amino acid; captodative effect; late‐stage functionalization; radical coupling; thianthrenium salt.
© 2024 The Author(s). Advanced Science published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- a) Cooper B. M., Legre J., O’ Donovan D. H., Ölwegård Halvarssonc M., Spring D. R., Chem. Soc. Rev. 2021, 50, 1480; - PubMed
- b) Fu C., Yu L., Miao Y., Liu X., Yu Z., Wei M., Acta. Pharm. Sin. B 2023, 13, 498; - PMC - PubMed
- c) Wang M.‐D., Hou D.‐Y., Lv G.‐T., Li R.‐X., Hu X.‐J., Wang Z.‐J., Zhang N.‐Y., Yi L., Xu W.‐H., Wang H., Biomaterials 2021, 278, 121139. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
