Genotype-specific effects of elamipretide in patients with primary mitochondrial myopathy: a post hoc analysis of the MMPOWER-3 trial
- PMID: 39574155
- PMCID: PMC11583740
- DOI: 10.1186/s13023-024-03421-5
Genotype-specific effects of elamipretide in patients with primary mitochondrial myopathy: a post hoc analysis of the MMPOWER-3 trial
Abstract
Background: As previously published, the MMPOWER-3 clinical trial did not demonstrate a significant benefit of elamipretide treatment in a genotypically diverse population of adults with primary mitochondrial myopathy (PMM). However, the prespecified subgroup of subjects with disease-causing nuclear DNA (nDNA) pathogenic variants receiving elamipretide experienced an improvement in the six-minute walk test (6MWT), while the cohort of subjects with mitochondrial DNA (mtDNA) pathogenic variants showed no difference versus placebo. These published findings prompted additional genotype-specific post hoc analyses of the MMPOWER-3 trial. Here, we present these analyses to further investigate the findings and to seek trends and commonalities among those subjects who responded to treatment, to build a more precise Phase 3 trial design for further investigation in likely responders.
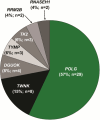
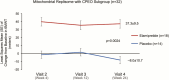
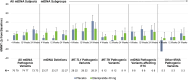
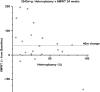
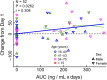
Results: Subjects with mtDNA pathogenic variants or single large-scale mtDNA deletions represented 74% of the MMPOWER-3 population, with 70% in the mtDNA cohort having either single large-scale mtDNA deletions or MT-TL1 pathogenic variants. Most subjects in the nDNA cohort had pathogenic variants in genes required for mtDNA maintenance (mtDNA replisome), the majority of which were in POLG and TWNK. The mtDNA replisome post-hoc cohort displayed an improvement on the 6MWT, trending towards significant, in the elamipretide group when compared with placebo (25.2 ± 8.7 m versus 2.0 ± 8.6 m for placebo group; p = 0.06). The 6MWT results at week 24 in subjects with replisome variants showed a significant change in the elamipretide group subjects who had chronic progressive external ophthalmoplegia (CPEO) (37.3 ± 9.5 m versus - 8.0 ± 10.7 m for the placebo group; p = 0.0024). Pharmacokinetic (exposure-response) analyses in the nDNA cohort showed a weak positive correlation between plasma elamipretide concentration and 6MWT improvement.
Conclusions: Post hoc analyses indicated that elamipretide had a beneficial effect in PMM patients with mtDNA replisome disorders, underscoring the importance of considering specific genetic subtypes in PMM clinical trials. These data serve as the foundation for a follow-up Phase 3 clinical trial (NuPOWER) which has been designed as described in this paper to determine the efficacy of elamipretide in patients with mtDNA maintenance-related disorders.
Classification of evidence: Class I CLINICALTRIALS.
Gov identifier: NCT03323749.
Keywords: Elamipretide; Mitochondria; MtDNA maintenance; MtDNA multiple deletions; PMM; Replisome.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: MMPOWER-3 was conducted in accordance with international ethics guidelines, including the Declaration of Helsinki, Council for International Organizations of Medical Sciences International Ethical Guidelines, ICH GCP guidelines, and all applicable laws and regulations. The trial was approved by institutional review boards, and all subjects provided written informed consent. Consent for publication: Not applicable.
Figures

References
-
- Mancuso M, McFarland R, Klopstock T, Hirano M, (2017) Consortium on Trial Readiness in Mitochondrial Myopathies. International Workshop: outcome measures and clinical trial readiness in primary mitochondrial myopathies in children and adults. Consensus recommendations. 16–18 November 2016, Rome, Italy. Neuromuscul Disord; 27(12):1126–37. - PMC - PubMed
-
- National Organization for Rare Disorders. Mitochondrial Myopathy (MM). 2016. https://rarediseases.org/physician-guide/mitochondrial-myopathy/. Accessed May 15, 2024.
-
- Tarnopolsky M. Exercise testing as a diagnostic entity in mitochondrial myopathies. Mitochondrion. 2004;4(5–6):529–42. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

