Stereospecific Properties and Intracellular Transport of Novel Intrinsically Fluorescent Neurosteroids
- PMID: 39574303
- PMCID: PMC11892034
- DOI: 10.1021/acschemneuro.4c00571
Stereospecific Properties and Intracellular Transport of Novel Intrinsically Fluorescent Neurosteroids
Abstract
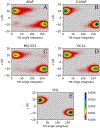
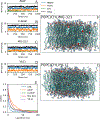
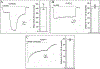
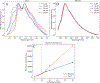
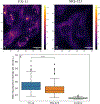
Allopregnanolone (AlloP) is an example of neuroactive steroids (NAS), which is a potent allosteric activator of the γ-aminobutyric acid A (GABAA) receptor. The mechanisms underlying the biological activity of AlloP and other NAS are only partially understood. Here, we present intrinsically fluorescent analogs of AlloP (MQ-323) and its 3β-epimer, epi-allopregnanolone (E-AlloP) (YX-11), and show, by a combination of spectroscopic and computational studies, that these analogs mimic the membrane properties of AlloP and E-AlloP very well. We found stereospecific differences in the orientation and dynamics of the NAS as well as in their impact on membrane permeability. However, all NAS are unable to condense the lipid bilayer, in stark contrast to cholesterol. Using Förster resonance energy transfer (FRET) and electrophysiological measurements, we show that MQ-323 but not YX-11 binds at the intersubunit site of the ELICα1GABAA receptor and potentiates GABA-induced receptor currents. In aqueous solvents, YX-11 forms aggregates at much lower concentrations than MQ-323, and loading both analogs onto cyclodextrin allows for their uptake by human astrocytes, where they become enriched in lipid droplets (LDs), as shown by quantitative fluorescence microscopy. Trafficking of the NAS analogs is stereospecific, as uptake and lipid droplet targeting is more pronounced for YX-11 compared to MQ-323. In summary, we present novel minimally modified analogs of AlloP and E-AlloP, which enable us to reveal stereospecific membrane properties, allosteric receptor activation, and intracellular transport of these neurosteroids. Our fluorescence design strategy will be very useful for the analysis of other NAS in the future.
Keywords: allopregnanolone; astrocytes; fluorescence; microscopy; probes; trafficking.
Conflict of interest statement
Author Competing Interests
The authors declare no competing financial interest.
Figures


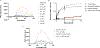

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

