Suppression of Hepatocyte Ferroptosis via USP19-Mediated Deubiquitination of SLC7A11 in Ischemia-Free Liver Transplantation
- PMID: 39574305
- PMCID: PMC11809379
- DOI: 10.1002/advs.202406200
Suppression of Hepatocyte Ferroptosis via USP19-Mediated Deubiquitination of SLC7A11 in Ischemia-Free Liver Transplantation
Abstract
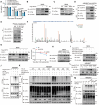
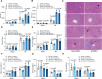
Ischemia-free liver transplantation (IFLT) is developed as a novel clinical approach to avoid ischemia-reperfusion injury (IRI). This study aims to identify the most distinguished programmed cell death pathway in grafts undergoing IFLT versus conventional liver transplantation (CLT) and to explore the underlying mechanism. Ferroptosis is the most distinct programmed cell death form between IFLT and CLT grafts. Among various cell death inhibitors, the ferroptosis inhibitor (Ferrostain-1) is the most effective one to prevent hepatocytes from damage induced by oxygen deprivation/reoxygenation (OGD/R). Hepatocyte ferroptosis is significantly alleviated in IFLT versus CLT grafts in both human beings and pigs. Ubiquitination enzyme screening identifies augmented amounts of ubiquitin-specific protease 19 (USP19) in IFLT versus CLT grafts. The upregulation of USP19 in the grafts is correlated with reduced pathological Suzuki's score, lower post-transplant peak liver enzyme level, and less early allograft dysfunction in liver transplant recipients. USP19 overexpression mitigates post-transplant liver injury in mice. Mechanistically, USP19 inhibits the degradation of solute carrier family 7 member 11 (SLC7A11) by removing its K63-linked ubiquitin chains. Notably, USP19 overexpression reduces ferroptosis and IRI in a SLC7A11-dependent manner in mice. Collectively, USP19-mediated suppression of hepatocyte ferroptosis via deubiquitinating SLC7A11 is a key mechanism by which IFLT abrogates graft IRI.
Keywords: ferroptosis; ischemia reperfusion injury; liver transplantation; ubiquitin specific protease 19.
© 2024 The Author(s). Advanced Science published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
-
- Peralta C., Jiménez‐Castro M. B., Gracia‐Sancho J., J. Hepatol. 2013, 59, 1094. - PubMed
-
- Ito T., Naini B. V., Markovic D., Aziz A., Younan S., Lu M., Hirao H., Kadono K., Kojima H., DiNorcia J. 3rd, Agopian V. G., Yersiz H., Farmer D. G., Busuttil R. W., Kupiec‐Weglinski J. W., Kaldas F. M., Am. J. Transplant. 2021, 21, 614. - PubMed
-
- de Meijer V. E., Fujiyoshi M., Porte R. J., J. Hepatol. 2019, 70, 203. - PubMed
-
- Czigany Z., Lurje I., Tolba R. H., Neumann U. P., Tacke F., Lurje G., Liver Int. 2019, 39, 228. - PubMed
MeSH terms
Substances
Grants and funding
- 81970564/National Natural Science Foundation of China
- 82070670/National Natural Science Foundation of China
- 82170663/National Natural Science Foundation of China
- 82370664/National Natural Science Foundation of China
- 82300744/National Natural Science Foundation of China
- 82372187/National Natural Science Foundation of China
- 2023B1212060020/Guangdong Provincial Key Laboratory of Organ Medicine
- 2020A0505020003/Guangdong Provincial International Cooperation Base of Science and Technology (Organ Transplantation)
- 2024B1515040011/Science and Technology Program of Guangdong
- China Organ Transplantation Development Foundation
LinkOut - more resources
Full Text Sources
Medical
Research Materials
