Why Bestatin Prefers Human Carnosinase 2 (CN2) to Human Carnosinase 1 (CN1)
- PMID: 39574306
- PMCID: PMC11626516
- DOI: 10.1021/acs.jpcb.4c05571
Why Bestatin Prefers Human Carnosinase 2 (CN2) to Human Carnosinase 1 (CN1)
Abstract
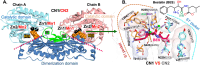
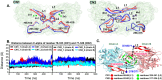
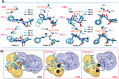
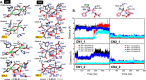
Human carnosinases (CNs) are Xaa-His metal-ion-activated aminopeptidases that break down bioactive carnosine and other histidine-containing dipeptides. Carnosine is a bioactive peptide found in meat and prevalently used as a supplement and in functional food formulation. Nonetheless, carnosine is digested by CNs rapidly after ingestion. CNs have two isoforms (carnosinase 1 (CN1) and carnosinase 2 (CN2)), where CN1 is the main player in carnosine digestion. CNs contain a catalytic metal ion pair (Zn2+ for CN1 and Mn2+ for CN2) and two subpockets (S1 and S1' pockets) to accommodate a substrate. Bestatin (BES) has been reported to be active for CN2; however, its inhibition ability for CN1 has remained under debate, because the underlying mechanism remains unclear. This information is important for designing novel CN1-selective inhibitors for proliferating carnosine after ingestion. Thus, molecular dynamics (MD) simulations were performed to explore the binding mechanism of BES to both CN1 and CN2. The binding of BES-CN1 and BES-CN2 was studied in comparison. The results indicated that BES could bind both CNs with different degrees of binding affinity. BES prefers CN2 because: (1) its aryl terminus is trapped by Y197 in an S1 pocket; (ii) the BES polar backbone is firmly bound by catalytic Mn2+ ions; and (iii) the S1' pocket can shrink to accommodate the isopropyl end of BES. In contrast, the high mobility of the aryl end and the complete loss of metal-BES interactions in CN1 cause a loose BES binding. Seemingly, polar termini were required for a good CN1 inhibitor.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


Similar articles
-
Exploring the structural and dynamic differences between human carnosinase I (CN1) and II (CN2).Proteins. 2023 Jun;91(6):822-830. doi: 10.1002/prot.26469. Epub 2023 Jan 24. Proteins. 2023. PMID: 36637795
-
Mechanistic insights on anserine hydrolyzing activities of human carnosinases.Biochim Biophys Acta Gen Subj. 2023 Mar;1867(3):130290. doi: 10.1016/j.bbagen.2022.130290. Epub 2022 Dec 16. Biochim Biophys Acta Gen Subj. 2023. PMID: 36529243
-
Structural basis for substrate recognition and hydrolysis by mouse carnosinase CN2.J Biol Chem. 2008 Oct 3;283(40):27289-99. doi: 10.1074/jbc.M801657200. Epub 2008 Jun 12. J Biol Chem. 2008. PMID: 18550540
-
Carnosinases, their substrates and diseases.Molecules. 2014 Feb 21;19(2):2299-329. doi: 10.3390/molecules19022299. Molecules. 2014. PMID: 24566305 Free PMC article. Review.
-
Human carnosinases: A brief history, medicinal relevance, and in silico analyses.Drug Discov Today. 2024 Feb;29(2):103860. doi: 10.1016/j.drudis.2023.103860. Epub 2023 Dec 19. Drug Discov Today. 2024. PMID: 38128717 Review.
References
-
- Davies K. M.; Bohic S.; Carmona A.; Ortega R.; Cottam V.; Hare D. J.; Finberg J. P.; Reyes S.; Halliday G. M.; Mercer J. F.; Double K. L. Copper pathology in vulnerable brain regions in Parkinson’s disease. Neurobiol. Aging 2014, 35 (4), 858–866. 10.1016/j.neurobiolaging.2013.09.034. - DOI - PubMed
-
From NLM.
-
- Basun H.; Forssell L. G.; Wetterberg L.; Winblad B. Metals and trace elements in plasma and cerebrospinal fluid in normal aging and Alzheimer’s disease. J. Neural Transm.: Parkinson’s Dis. Dementia Sect. 1991, 3 (4), 231–258. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

