Exercise-Activated mPFC Tri-Synaptic Pathway Ameliorates Depression-Like Behaviors in Mouse
- PMID: 39574315
- PMCID: PMC11744721
- DOI: 10.1002/advs.202408618
Exercise-Activated mPFC Tri-Synaptic Pathway Ameliorates Depression-Like Behaviors in Mouse
Abstract
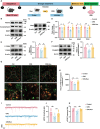
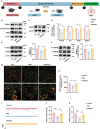
Exercise is considered as playing a pivotal role in the modulation of emotional responses. However, a precise circuit that mediates the effects of exercise on depression have yet to be elucidated. Here, a molecularly defined tri-synaptic pathway circuit is identified that correlates motor inputs with antidepressant effects. With this pathway, initial inputs from neurons within the dorsal root ganglia (DRG) project to excitatory neurons in the gracile nucleus (GR), which in turn connect with 5-HTergic neurons in the dorsal raphe nucleus (DRN), eventually coursing to excitatory pyramidal neurons within the medial prefrontal cortex (mPFC). Exercise activates this pathway, with the result that depressive- and anxiety-like behaviors in mice are significantly reduced. In addition, it is found that exercise may exert antidepressant effects through regulating synaptic plasticity within this tri-synaptic pathway. These findings reveal a hindbrain-to-forebrain neuronal circuit that specifically modulates depression and provides a potential mechanism for the antidepressant effects of exercise.
Keywords: depression; dorsal raphe nucleus; exercise; medial prefrontal cortex; synaptic plasticity.
© 2024 The Author(s). Advanced Science published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

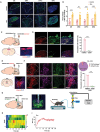
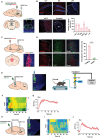
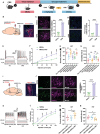
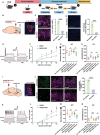
References
-
- Nagy C., Maitra M., Tanti A., Suderman M., Théroux J. F., Davoli M. A., Perlman K., Yerko V., Wang Y. C., Tripathy S. J., Pavlidis P., Mechawar N., Ragoussis J., Turecki G., Nat. Neurosci. 2020, 23, 771. - PubMed
-
- Papakostas G. I., Ionescu D. F., Mol. Psychiatry 2015, 20, 1142. - PubMed
-
- Otte C., Gold S. M., Penninx B. W., Pariante C. M., Etkin A., Fava M., Mohr D. C., Schatzberg A. F., Nat. Rev. Dis. Primers 2016, 2, 16065. - PubMed
-
- Fellinger M., Waldhor T., Serretti A., Hinterbuchinger B., Pruckner N., Konig D., Gmeiner A., Vyssoki S., Vyssoki B., Fugger G., J. Affect Disord. 2022, 296, 111. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
