A comparison of natalizumab's effects on SDMT between pediatric-onset and adult-onset multiple sclerosis patients
- PMID: 39574510
- PMCID: PMC11579921
- DOI: 10.3389/fneur.2024.1475161
A comparison of natalizumab's effects on SDMT between pediatric-onset and adult-onset multiple sclerosis patients
Abstract
Background: Pediatric-onset multiple sclerosis (POMS) patients often exhibit a wide range of cognitive deficits. Therefore, therapeutic approaches should aim not only to prevent cognitive decline but also to promote cognitive improvement.
Objective: This study aimed to explore the effects of natalizumab (NTZ) on cognitive function, as measured by the Symbol Digit Modalities Test (SDMT), in both POMS and adult-onset multiple sclerosis (AOMS) patients.
Method: A total of 63 patients (34 AOMS and 29 POMS) were enrolled in this retrospective, single-center study. Patients were clinically and radiologically assessed every 6 months, and they completed the SDMT at baseline and after at least 24 months of follow-up. SDMT values were reported as corrected values (cSDMT) and z-scores (zSDMT). Annualized cSDMT and zSDMT scores were calculated by dividing the change in scores by the length of the follow-up period (expressed in years).
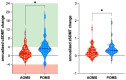
Results: Both POMS and AOMS groups showed improvement in annualized cSDMT and zSDMT scores, but the improvement was significantly greater in the POMS group compared to the AOMS group (+3.85 ± 4.32 vs. +1.76 ± 2.80, p = 0.010 for cSDMT; 0.41 ± 0.40 vs. 0.25 ± 0.34, p = 0.026 for zSDMT). After re-baselining at 6 months, 93% of POMS patients (27 patients) and 85.3% of AOMS patients (29 patients, p = 0.84) achieved NEDA-3 (no evidence of disease activity). The NEDA-3 status, along with clinical and demographic parameters at baseline, did not account for the observed SDMT improvement.
Conclusion: The favorable clinical, radiological, and neuropsychological outcomes observed in this study support the use of natalizumab as a viable treatment option in POMS.
Keywords: Symbol Digit Modalities Test; multiple sclerosis; natalizumab; neuropsychological outcome; pediatric-onset multiple sclerosis.
Copyright © 2024 Puthenparampil, Scialpi, Gaggiola, Zanotelli, Miscioscia, Berardi, Riccardi, Nosadini, Sartori, Perini, Rinaldi and Gallo.
Conflict of interest statement
M.Pu. report grants from Almirall, Teva, Sanofi Genzyme, Merck Serono, Biogen Italy, Novartis; consultancy for Novartis, Biogen Italy, Sanofi Genzyme; board membership Sanofi Genzyme, Novartis, Biogen Italy. P.P. reports grants from Almirall, Teva, Sanofi Genzyme, Merck Serono, Biogen Italy, Novartis, Roche; consultancy for Novartis, Biogen Italy, Sanofi Genzyme, Roche. R.F. report grants from Almirall, Teva, Sanofi Genzyme, Merck Serono, Biogen Italy, Novartis; consultancy for Novartis, Biogen Italy, Sanofi Genzyme. P.G. reports a grant from Almirall, Teva, Sanofi Genzyme, Merck Serono, Biogen Italy, Novartis, Roche, Bristol Myers Squibb; consultancy for Novartis, Biogen Italy, Sanofi Genzyme, Roche, Bristol Myers Squibb; board membership Sanofi Genzyme, Novartis, Biogen Italy, Roche, Merck Serono, Bristol Myers Squibb. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures
References
LinkOut - more resources
Full Text Sources

