This is a preprint.
Phenotypic plasticity in bacterial elongation among closely related species
- PMID: 39574579
- PMCID: PMC11581012
- DOI: 10.1101/2024.11.07.622495
Phenotypic plasticity in bacterial elongation among closely related species
Update in
-
Phenotypic plasticity in cell elongation among closely related bacterial species.Nat Commun. 2025 Jun 2;16(1):5099. doi: 10.1038/s41467-025-60005-y. Nat Commun. 2025. PMID: 40456757 Free PMC article.
Abstract
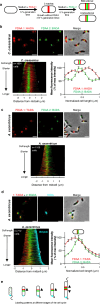
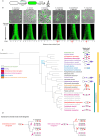
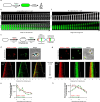
Cell elongation is a fundamental component of the bacterial cell cycle and has been studied over many decades, in part owing to its mechanisms being a target of numerous antibiotic classes. While several distinct modes of cell elongation have been described, these studies have largely relied on a handful of model bacterial species. Therefore, we have a limited view of the diversity of cell elongation approaches that are employed by bacteria, and how these vary in response to evolutionary and environmental influences. Here, by employing fluorescent D-amino acids (FDAAs) to track the spatiotemporal dynamics of elongation, we reveal previously unsuspected diversity of elongation modes among closely related species of the Caulobacteraceae, with species-specific combinations of dispersed, midcell and polar elongation that can be either unidirectional or bidirectional. Using genetic, cell biology, and phylogenetic approaches, we demonstrate that evolution of unidirectional-midcell elongation is accompanied by changes in the localization pattern of the peptidoglycan synthase PBP2 and infer that elongation complexes display a high degree of phenotypic plasticity, both among the Caulobacteraceae and more widely among the Alphaproteobacteria. Demonstration that even closely related bacterial species employ highly distinct mechanisms of cell elongation reshapes our understanding of the evolution and regulation of bacterial cell growth, with broad implications for bacterial morphology, adaptation, and antibiotic resistance.
Keywords: Caulobacter; Caulobacteraceae; Elongation modes; Peptidoglycan; midcell elongation.
Conflict of interest statement
Competing interests The authors declare no competing interests.
Figures



References
-
- Egan A. J., Errington J. & Vollmer W. Regulation of peptidoglycan synthesis and remodelling. Nature Reviews Microbiology 18, 446–460 (2020). - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
