This is a preprint.
Selective Enhancement of the Interneuron Network and Gamma-Band Power via GluN2C/GluN2D NMDA Receptor Potentiation
- PMID: 39574703
- PMCID: PMC11580944
- DOI: 10.1101/2024.11.05.622179
Selective Enhancement of the Interneuron Network and Gamma-Band Power via GluN2C/GluN2D NMDA Receptor Potentiation
Update in
-
Selective enhancement of the interneuron network and gamma-band power via GluN2C/GluN2D NMDA receptor potentiation.J Physiol. 2025 Jul;603(14):4027-4049. doi: 10.1113/JP288343. Epub 2025 Jun 25. J Physiol. 2025. PMID: 40560934 Free PMC article.
Abstract
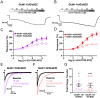
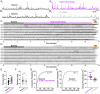
N-methyl-D-aspartate receptors (NMDARs) comprise a family of ligand-gated ionotropic glutamate receptors that mediate a slow, calcium-permeable component to excitatory neurotransmission. The GluN2D subunit is enriched in GABAergic inhibitory interneurons in cortical tissue. Diminished levels of GABAergic inhibition contribute to multiple neuropsychiatric conditions, suggesting that enhancing inhibition may have therapeutic utility, thus making GluN2D modulation an attractive drug target. Here, we describe the actions of a GluN2C/GluN2D-selective positive allosteric modulator (PAM), (+)-EU1180-453, which has improved drug-like properties such as increased aqueous solubility compared to the first-in-class GluN2C/GluN2D-selective prototypical PAM (+)-CIQ. (+)-EU1180-453 doubles the NMDAR response at lower concentrations (< 10 μM) compared to (+)-CIQ, and produces a greater degree of maximal potentiation at 30 μM. Using in vitro electrophysiological recordings, we show that (+)-EU1180-453 potentiates triheteromeric NMDARs containing at least one GluN2C or GluN2D subunit, and is active at both exon5-lacking and exon5-containing GluN1 splice variants. (+)-EU1180-453 increases glutamate efficacy for GluN2C/GluN2D-containing NMDARs by both prolonging the deactivation time and potentiating the peak response amplitude. We show that (+)-EU1180-453 selectively increases synaptic NMDAR-mediated charge transfer onto P11-15 CA1 stratum radiatum hippocampal interneurons, but is without effect on CA1 pyramidal cells. This increased charge transfer enhances inhibitory output from GABAergic interneurons onto CA1 pyramidal cells in a GluN2D-dependent manner. (+)-EU1180-453 also shifts excitatory-to-inhibitory coupling towards increased inhibition and produces enhanced gamma band power from carbachol-induced field potential oscillations in hippocampal slices. Thus, (+)-EU1180-453 can enhance overall circuit inhibition, which could prove therapeutically useful for the treatment of anxiety, depression, schizophrenia, and other neuropsychiatric disorders.
Significance statement: Interneuron dysfunction and diminished GABAergic inhibition in neocortical and hippocampal circuits remains a prominent molecular hypothesis for neuropsychiatric diseases including anxiety, depression, and schizophrenia. Pharmacological agents that boost GABA receptor function have shown utility in various forms of depression and treating symptoms of schizophrenia. Cortical GABAergic interneurons, unlike their excitatory pyramidal cell counterparts, are enriched for the GluN2D subunit of the NMDA receptor. Thus, GluN2D subunit-selective modulation could be a useful therapeutic tool to enhance local inhibition, improving the prognosis for neuropsychiatric diseases for which interneuron dysfunction is prominent and causal to circuit aberration.
Conflict of interest statement
Conflict of interest: D.C.L., H.Y., M.P.E., N.S.A., and S.F.T. are co-inventors of Emory-owned intellectual property. S.F.T. is a member of the SAB for Sage Therapeutics, Eumentis Therapeutics, Neurocrine, the GRIN2B Foundation, the CureGRIN Foundation, and CombinedBrain. S.F.T. is a consultant for GRIN Therapeutics. H.Y. is the PI on a research grant from Sage Therapeutics and GRIN Therapeutics to Emory. S.F.T. is PI on a research grant from GRIN Therapeutics to Emory. S.F.T. is cofounder of NeurOp, Inc. and Agrithera. D.C.L. and S.F.T. are on the Board of Directors for NeurOp. T.A.B. is a member of the SAB for GRIN2B Foundation, CureGRIN Foundation and GRIN Therapeutics; all remuneration has been made to his department.
Figures




References
-
- Akazawa C., Shigemoto R., Bessho Y., Nakanishi S., and Mizuno N. (1994). Differential expression of five N-methyl-D-aspartate receptor subunit mRNAs in the cerebellum of developing and adult rats. J Comp Neurol 347, 150–160. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
