This is a preprint.
Personalized azithromycin treatment rules for children with watery diarrhea using machine learning
- PMID: 39574840
- PMCID: PMC11581062
- DOI: 10.1101/2024.10.27.24316217
Personalized azithromycin treatment rules for children with watery diarrhea using machine learning
Update in
-
Personalized azithromycin treatment rules for children with watery diarrhea using machine learning.Nat Commun. 2025 Jul 1;16(1):5968. doi: 10.1038/s41467-025-60682-9. Nat Commun. 2025. PMID: 40592849 Free PMC article. Clinical Trial.
Abstract
Introduction: We used machine learning to identify novel strategies to target azithromycin to the children with watery diarrhea who are most likely to benefit.
Methods: Using data from a randomized trial of azithromycin for watery diarrhea, we developed personalized treatment rules given sets of diagnostic, child, and clinical characteristics, employing a robust ensemble machine learning-based procedure. For each rule, we estimated the proportion treated under the rule and the average benefits of treatment.
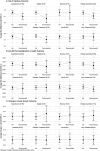
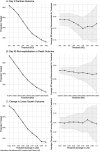
Results: Among 6,692 children, treatment was recommended on average for approximately one third of children. The risk of diarrhea on day 3 was 10.1% lower (95% CI: 5.4, 14.9) with azithromycin compared to placebo among children recommended for treatment. For day 90 re-hospitalization and death, risk was 2.4% lower (95% CI: 0.6, 4.1) with azithromycin compared to placebo among those recommended for treatment. While pathogen diagnostics were strong determinants of azithromycin effects on diarrhea duration, host characteristics were more relevant for predicting benefits for re-hospitalization or death.
Conclusion: The ability of host characteristics to predict which children benefit from azithromycin with respect to the most severe outcomes suggests appropriate targeting of antibiotic treatment among children with watery diarrhea may be possible without access to pathogen diagnostics.
Conflict of interest statement
Competing interests The authors have no competing interests as defined by Nature Research, or other interests that might be perceived to influence the interpretation of the article.
Figures

References
-
- Troeger C, Blacker BF, Khalil IA, Rao PC, Cao S, Zimsen SR, et al. Estimates of the global, regional, and national morbidity, mortality, and aetiologies of diarrhoea in 195 countries: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Infect Dis. 2018. Nov 1;18(11):1211–28. - PMC - PubMed
-
- Platts-Mills JA, Liu J, Rogawski ET, Kabir F, Lertsethtakarn P, Siguas M, et al. Use of quantitative molecular diagnostic methods to assess the aetiology, burden, and clinical characteristics of diarrhoea in children in low-resource settings: a reanalysis of the MAL-ED cohort study. Lancet Glob Health. 2018. Dec 1;6(12):e1309–18. - PMC - PubMed
-
- Kotloff KL, Nataro JP, Blackwelder WC, Nasrin D, Farag TH, Panchalingam S, et al. Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the Global Enteric Multicenter Study, GEMS): a prospective, case-control study. The Lancet. 2013. Jul 20;382(9888):209–22. - PubMed
-
- Rogawski ET, Liu J, Platts-Mills JA, Kabir F, Lertsethtakarn P, Siguas M, et al. Use of quantitative molecular diagnostic methods to investigate the effect of enteropathogen infections on linear growth in children in low-resource settings: longitudinal analysis of results from the MAL-ED cohort study. Lancet Glob Health. 2018. Dec 1;6(12):e1319–28. - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
