Several methods for assessing research waste in reviews with a systematic search: a scoping review
- PMID: 39575170
- PMCID: PMC11580664
- DOI: 10.7717/peerj.18466
Several methods for assessing research waste in reviews with a systematic search: a scoping review
Abstract
Background: Research waste is present in all study designs and can have significant consequences for science, including reducing the reliability of research findings and contributing to the inefficient use of resources. Estimates suggest that as much as 85% of all biomedical research is wasted. However, it is uncertain how avoidable research waste is assessed in specific types of study designs and what methods could be used to examine different aspects of research waste. We aimed to investigate which methods, systematic reviews, scoping reviews, and overviews of reviews discussing research waste, have used to assess avoidable research waste.
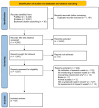
Materials and methods: We published a protocol in the Open Science Framework prospectively (https://osf.io/2fbp4). We searched PubMed and Embase with a 30-year limit (January 1993-August 2023). The concept examined was how research waste and related synonyms (e.g., unnecessary, redundant, duplicate, etc.) were assessed in reviews with a systematic search: systematic, scoping, or overviews of reviews. We extracted data on the method used in the review to examine for research waste and for which study design this method was applied.
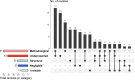
Results: The search identified 4,285 records of which 93 reviews with systematic searches were included. The reviews examined a median of 90 (range 10-6,781) studies, where the study designs most commonly included were randomized controlled trials (48%) and systematic reviews (33%). In the last ten years, the number of reports assessing research waste has increased. More than 50% of examined reviews reported evaluating methodological research waste among included studies, typically using tools such as one of Cochrane Risk of Bias tools (n = 8) for randomized controlled trials or AMSTAR 1 or 2 (n = 12) for systematic reviews. One fourth of reviews assessed reporting guideline adherence to e.g., CONSORT (n = 4) for randomized controlled trials or PRISMA (n = 6) for systematic reviews.
Conclusion: Reviews with systematic searches focus on methodological quality and reporting guideline adherence when examining research waste. However, this scoping review revealed that a wide range of tools are used, which may pose difficulties in comparing examinations and performing meta-research. This review aids researchers in selecting methodologies and contributes to the ongoing discourse on optimizing research efficiency.
Keywords: Duplicate efforts; Evidence-based medicine; Redundant research; Research waste; Scoping review; Systematic review.
©2024 Rosengaard et al.
Conflict of interest statement
The authors declare there are no competing interests.
Figures

Similar articles
-
The future of Cochrane Neonatal.Early Hum Dev. 2020 Nov;150:105191. doi: 10.1016/j.earlhumdev.2020.105191. Epub 2020 Sep 12. Early Hum Dev. 2020. PMID: 33036834
-
Healthcare outcomes assessed with observational study designs compared with those assessed in randomized trials: a meta-epidemiological study.Cochrane Database Syst Rev. 2024 Jan 4;1(1):MR000034. doi: 10.1002/14651858.MR000034.pub3. Cochrane Database Syst Rev. 2024. PMID: 38174786 Free PMC article.
-
A Critical Analysis of Reporting in Systematic Reviews and Meta-Analyses in the Peyronie's Disease Literature.J Sex Med. 2022 Apr;19(4):629-640. doi: 10.1016/j.jsxm.2022.01.008. Epub 2022 Feb 15. J Sex Med. 2022. PMID: 35177375 Free PMC article.
-
Meta-research evaluating redundancy and use of systematic reviews when planning new studies in health research: a scoping review.Syst Rev. 2022 Nov 15;11(1):241. doi: 10.1186/s13643-022-02096-y. Syst Rev. 2022. PMID: 36380367 Free PMC article.
-
Characteristics, methodological, and reporting quality of scoping reviews published in nursing journals: A systematic review.J Nurs Scholarsh. 2023 Jul;55(4):874-885. doi: 10.1111/jnu.12861. Epub 2022 Dec 9. J Nurs Scholarsh. 2023. PMID: 36494752
References
-
- Agbadjé TT, Riganti P, Adisso ÉL, Adekpedjou R, Boucher A, Nunciaroni AT, Franco JVA, Yanzi MVR, Légaré F. Are shared decision making studies well enough described to be replicated? Secondary analysis of a Cochrane systematic review. PLOS ONE. 2022;17:e0265401. doi: 10.1371/journal.pone.0265401. - DOI - PMC - PubMed
-
- Andaur Navarro CL, Damen JAA, Takada T, Nijman SWJ, Dhiman P, Ma J, Collins GS, Bajpai R, Riley RD, Moons KGM, Hooft L. Completeness of reporting of clinical prediction models developed using supervised machine learning: a systematic review. BMC Medical Research Methodology. 2022;22:12. doi: 10.1186/s12874-021-01469-6. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

