MALAT1 promotes colonic epithelial cell apoptosis and pyroptosis by sponging miR-22-3p to enhance NLRP3 expression
- PMID: 39575175
- PMCID: PMC11580658
- DOI: 10.7717/peerj.18449
MALAT1 promotes colonic epithelial cell apoptosis and pyroptosis by sponging miR-22-3p to enhance NLRP3 expression
Abstract
Background: Colonic epithelial cell apoptosis and pyroptosis had a close relationship with the pathological progression of ulcerative colitis (UC). LncRNA play a crucial role in the progression of UC. However, the role of the lncRNA MALAT1 in colonic epithelial cell apoptosis and pyroptosis remains unclear.
Methods: UC colitis cell model was established through lipopolysaccharide (LPS) treatment. MiR-22-3p and MALAT1 expression in fetal human colon (FHC) cells were analyzed by qRT-PCR. Proliferation and apoptosis of FHCs were measured using CCK-8 assay and flow cytometry, respectively. Pyroptosis indicators including interleukin (IL)-1β, IL-18, tumor necrosis factor-α (TNF-α), NLR family pyrin domain containing 3 (NLRP3), caspase-1, and N-gasdermin D (N-GSDMD) in FHCs were detected using ELISA, qRT-PCR, western blotting, and immunofluorescence.
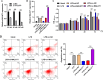
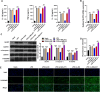
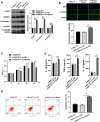
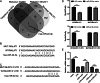
Results: In this study, apoptosis was facilitated, IL-1β, IL-18, and TNF-α levels were enhanced, NLRP3, caspase-1, N-GSDMD protein were increased, and MALAT1 expression was markedly increased in LPS-treated FHCs (LTFs). MALAT1 knockdown remarkably facilitated proliferation and suppressed apoptosis, reduced IL-1β, IL-18, and TNF-α levels, and decreased the protein of NLRP3, caspase-1, N-GSDMD. Furthermore, NLRP3 overexpression remarkably reversed the effect of MALAT1-downexpression in LTFs. In addition, miR-22-3p could bind with MALAT1 and NLRP3 3' UTR. Furthermore, miR-22-3p inhibition remarkably reversed the effect of MALAT1 overexpression in LTFs.
Conclusions: These findings suggest that MALAT1 represents a promising therapeutic target for the treatment of UC by modulating the miR-22-3p/NLRP3 pathway, potentially leading to novel strategies for reducing inflammation and cell death in the colon.
Keywords: Fetal human colon cell; Inflammasome; Long non-coding RNAs; Proliferation.
© 2024 Yan et al.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
Similar articles
-
LncRNA MALAT1 promoted high glucose-induced pyroptosis of renal tubular epithelial cell by sponging miR-30c targeting for NLRP3.Kaohsiung J Med Sci. 2020 Sep;36(9):682-691. doi: 10.1002/kjm2.12226. Epub 2020 May 11. Kaohsiung J Med Sci. 2020. PMID: 32391974 Free PMC article.
-
[Electroacupuncture improves colonic mucosal barrier damage by regulating NLRP3/Caspase-1/GSDMD signaling pathway and inhibiting pyroptosis in ulcerative colitis mice].Zhen Ci Yan Jiu. 2025 Mar 25;50(3):277-286. doi: 10.13702/j.1000-0607.20240402. Zhen Ci Yan Jiu. 2025. PMID: 40103379 Chinese.
-
Lipopolysaccharide-induced bacterial infection model: microRNA-370-3p participates in the anti-infection response by targeting the macrophage TLR4-NLRP3 caspase-1 cellular pyroptosis pathway.Int J Immunopathol Pharmacol. 2024 Jan-Dec;38:3946320241272550. doi: 10.1177/03946320241272550. Int J Immunopathol Pharmacol. 2024. PMID: 39101927 Free PMC article.
-
Genetic and Epigenetic Regulation of the Innate Immune Response to Gout.Immunol Invest. 2023 Apr;52(3):364-397. doi: 10.1080/08820139.2023.2168554. Epub 2023 Feb 6. Immunol Invest. 2023. PMID: 36745138 Review.
-
The Association between the NLRP3 Inflammasome and Specific Long-Non Coding RNAs (lncRNAs) in Cancer; New Perspective and Summary of Recent Studies.Cell Biochem Biophys. 2025 Mar;83(1):147-158. doi: 10.1007/s12013-024-01494-4. Epub 2024 Sep 16. Cell Biochem Biophys. 2025. PMID: 39285156 Review.
Cited by
-
RNA-Seq analysis reveals the long noncoding RNAs associated with immunity in wild Myotis myotis bats.BMC Genomics. 2025 Apr 5;26(1):345. doi: 10.1186/s12864-025-11485-1. BMC Genomics. 2025. PMID: 40188093 Free PMC article.
-
Bone marrow mesenchymal stem cell-derived exosomes alleviate DSS-induced inflammatory bowel disease in mice through inhibiting intestinal epithelial cell pyroptosis via delivery of TSG-6.Front Immunol. 2025 Jun 30;16:1601591. doi: 10.3389/fimmu.2025.1601591. eCollection 2025. Front Immunol. 2025. PMID: 40661945 Free PMC article.
References
-
- Choy MC, Seah D, Faleck DM, Shah SC, Chao CY, An YK, Radford-Smith G, Bessissow T, Dubinsky MC, Ford AC, Churilov L, Yeomans ND, De Cruz PP. Systematic review and meta-analysis: optimal salvage therapy in acute severe ulcerative colitis. Inflammatory Bowel Diseases. 2019;25(7):1169–1186. doi: 10.1093/ibd/izy383. - DOI - PMC - PubMed
-
- Dai L, Zhang G, Cheng Z, Wang X, Jia L, Jing X, Wang H, Zhang R, Liu M, Jiang T, Yang Y, Yang M. Knockdown of LncRNA MALAT1 contributes to the suppression of inflammatory responses by up-regulating miR-146a in LPS-induced acute lung injury. Connective Tissue Research. 2018;59(6):581–592. doi: 10.1080/03008207.2018.1439480. - DOI - PubMed
-
- Eder P, Łodyga M, Gawron-Kiszka M, Dobrowolska A, Gonciarz M, Hartleb M, Kłopocka M, Małecka-Wojciesko E, Radwan P, Reguła J, Zagórowicz E, Banasiewicz T, Durlik M, Rydzewska G. Guidelines for the management of ulcerative colitis. Recommendations of the polish society of gastroenterology and the polish national consultant in gastroenterology. Przeglad Gastroenterologiczny. 2023;18(1):1–42. doi: 10.5114/pg.2023.125882. - DOI - PMC - PubMed
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

