The microbiome analysis of ripen grape berries supports the complex etiology of sour rot
- PMID: 39575185
- PMCID: PMC11578972
- DOI: 10.3389/fmicb.2024.1450443
The microbiome analysis of ripen grape berries supports the complex etiology of sour rot
Abstract
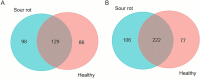
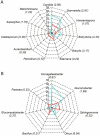
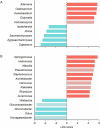
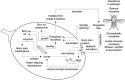
Sour rot (SR) is a grapevine disease complex that is not completely understood in its etiology and epidemiology. Recently, SR has received special attention due to its increasing economic importance due to crop losses and reduced wine quality. In this study, the fungal and bacterial microbiota of healthy (i.e., without rot symptoms) and rotten (i.e., exhibiting visual and olfactory SR symptoms) ripe bunches were characterized across 47 epidemics (39 vineyards in six Italian grape-growing areas) over three years. The 16S rRNA gene, ITS high-throughput amplicon sequencing, and quantitative PCR were used to assess the relative abundance and dynamic changes of microorganisms associated with SR. The estimators of genera richness of fungal communities within samples indicated a significantly different diversity between healthy and rotten bunches. For bacterial communities, the healthy and rotten bunches significantly differed in the total number of species, but not in abundance distribution across species. The bunch status (i.e., healthy and rotten) was a significant source of diversity (p < 0.01) when the community composition between samples was evaluated, indicating that microbiome composition varied between healthy and rotten bunches. In particular, healthy and rotten bunches shared 43.1 and 54.8% of fungal and bacterial genera, respectively; 31.3% (fungal) and 26.2% (bacterial) genera were associated with rotten bunches only. The yeast genera Zygosaccharomyces, Zygoascus, Saccharomycopsis, Issatchenkia, and Pichia and the bacterial genera Orbus, Gluconobacter, Komagataeibacter, Gluconacetobacter, and Wolbachia were strongly associated with bunches showing SR symptoms based on a linear discriminant analysis. These microorganisms have been associated with Drosophila insects in literature. The relationships between the microflora associated with SR-affected bunches and the roles of Drosophila in SR development need further investigation, which may open perspectives for more effective disease control.
Keywords: Enterobacteriaceae; Vitis vinifera; acetic acid bacteria; bunch microflora; high-throughput sequencing; minor grape rots; non-Saccharomyces yeasts.
Copyright © 2024 Brischetto, Rossi and Fedele.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Knowledge gaps on grape sour rot inferred from a systematic literature review.Front Plant Sci. 2024 Jul 3;15:1415379. doi: 10.3389/fpls.2024.1415379. eCollection 2024. Front Plant Sci. 2024. PMID: 39022603 Free PMC article.
-
The epiphytic microbiota of sour rot-affected grapes differs minimally from that of healthy grapes, indicating causal organisms are already present on healthy berries.PLoS One. 2019 Mar 27;14(3):e0211378. doi: 10.1371/journal.pone.0211378. eCollection 2019. PLoS One. 2019. PMID: 30917111 Free PMC article.
-
Changes in sour rotten grape berry microbiota during ripening and wine fermentation.Int J Food Microbiol. 2012 Mar 15;154(3):152-61. doi: 10.1016/j.ijfoodmicro.2011.12.029. Epub 2012 Jan 8. Int J Food Microbiol. 2012. PMID: 22277696
-
New insights into the ecological interaction between grape berry microorganisms and Drosophila flies during the development of sour rot.Microb Ecol. 2012 Aug;64(2):416-30. doi: 10.1007/s00248-012-0041-y. Epub 2012 Mar 22. Microb Ecol. 2012. PMID: 22438040
-
Sour rot-damaged grapes are sources of wine spoilage yeasts.FEMS Yeast Res. 2008 Nov;8(7):1008-17. doi: 10.1111/j.1567-1364.2008.00399.x. Epub 2008 Jun 12. FEMS Yeast Res. 2008. PMID: 18554306 Review.
Cited by
-
Temperature Requirements Can Affect the Microbial Composition Causing Sour Rot in Grapes.Environ Microbiol Rep. 2025 Feb;17(1):e70061. doi: 10.1111/1758-2229.70061. Environ Microbiol Rep. 2025. PMID: 39871424 Free PMC article.
References
-
- Anagnostou C., Dorsch M., Rohlfs M. (2010). Influence of dietary yeasts on Drosophila melanogaster life-history traits. Entomol. Exp. Appl. 136, 1–11. doi: 10.1111/j.1570-7458.2010.00997.x - DOI
-
- Barata A. (2011). Microbial ecology of sour rotten grapes and their influence onchemical and sensorial wine quality. Tese apresentada Para obtenc¸ ão do grau deDoutor em Engenharia Alimentar. Portugal: Instituto Superior de Agronomía, UniversidadTécnica de Lisboa, 6.
-
- Barata A., Campo E., Malfeito-Ferreira M., Loureiro V., Cacho J., Ferreira V. (2011). Analytical and sensorial characterization of the aroma of wines produced with sour rotten grapes using GC-O and GC-MS: identification of key aroma compounds. J. Agric. Food Chem. 59, 2543–2553. doi: 10.1021/jf104141f, PMID: - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

