Systematic evaluation of therapeutic effectiveness of Azvudine in treating COVID-19 hospitalized patients: a retrospective cohort study
- PMID: 39575306
- PMCID: PMC11578945
- DOI: 10.3389/fcimb.2024.1453234
Systematic evaluation of therapeutic effectiveness of Azvudine in treating COVID-19 hospitalized patients: a retrospective cohort study
Abstract
Background: Azvudine, a repurposed oral small molecule antiviral drug, has potential effects in combating the SARS-CoV-2 virus. However, studies on its clinical efficacy in patients with COVID-19 are still limited and controversial, and further research and validation are necessary.
Methods: A retrospective cohort study was conducted on COVID-19 patients who were hospitalized in the General Hospital of Central Theater Command from 1 December 2022 to 31 January 2023. We included 132 patients treated with Azvudine and 132 controls after screening and propensity score matching. The primary outcomes including all-cause mortality and a composite outcome of disease progression such as non-invasive respiratory support, invasive respiratory support, admission to intensive care unit (ICU), and death were compared.
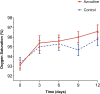
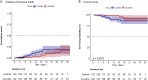
Results: Azvudine recipients had a much lower incidence rate of composite disease progression outcome than controls (13.9075/1000 person-days versus 25.7731/1000 person-days, P<0.05). Azvudine recipients also possessed a lower all-cause mortality rate than controls (2.6797/1000 person-days versus 8.5910/1000 person-days, P<0.01). Azvudine treatment significantly reduced the risk of composite disease progression (HR: 0.37, 95% CI: 0.16-0.84, P=0.017) and all-cause death (HR: 0.25, 95% CI: 0.08-0.81, P=0.021) after adjusting potential confounding factors such as age, sex, severity of COVID-19, complications, concomitant therapy, time from symptoms to treatment, and important laboratory indicators. The subgroup analyses of composite disease progression outcome and all-cause death indicated robustness of Azvudine's in treating COVID-19 patients in general.
Conclusion: Our study demonstrates that Azvudine has a significant positive impact on the clinical recovery of hospitalized patients with COVID-19. These findings provide important support for the use of Azvudine as a therapeutic option for COVID-19, given the current divergent views on its therapeutic efficacy and its importance in public health and medical care.
Keywords: Azvudine; COVID-19; all-cause death; composite disease progression outcome; retrospective cohort study.
Copyright © 2024 Xu, Huang, Yuan, Liu, Wang and Liu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Effectiveness of azvudine against severe outcomes among hospitalized COVID-19 patients in Xinjiang, China: a single-center, retrospective, matched cohort study.Expert Rev Anti Infect Ther. 2024 Jul;22(7):569-577. doi: 10.1080/14787210.2024.2362900. Epub 2024 Jun 27. Expert Rev Anti Infect Ther. 2024. PMID: 38822541
-
The Antiviral Efficacy and Safety of Azvudine in Hospitalized SARS-CoV-2 Infected Patients with Liver Diseases Based on a Multicenter, Retrospective Cohort Study.Adv Sci (Weinh). 2025 Apr;12(15):e2405679. doi: 10.1002/advs.202405679. Epub 2025 Feb 22. Adv Sci (Weinh). 2025. PMID: 39985372 Free PMC article.
-
Real-world effectiveness and safety of azvudine in hospitalized patients with SARS-CoV-2 infection: A multicenter, retrospective cohort study.J Infect. 2024 Dec;89(6):106355. doi: 10.1016/j.jinf.2024.106355. Epub 2024 Nov 17. J Infect. 2024. PMID: 39561881
-
Effectiveness and safety of azvudine in COVID-19: A systematic review and meta-analysis.PLoS One. 2024 Jun 13;19(6):e0298772. doi: 10.1371/journal.pone.0298772. eCollection 2024. PLoS One. 2024. PMID: 38870134 Free PMC article.
-
Ivermectin for preventing and treating COVID-19.Cochrane Database Syst Rev. 2021 Jul 28;7(7):CD015017. doi: 10.1002/14651858.CD015017.pub2. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 Jun 21;6:CD015017. doi: 10.1002/14651858.CD015017.pub3. PMID: 34318930 Free PMC article. Updated.
References
-
- Chen R., Guo Y., Deng S., Wang J., Gao M., Han H., et al. . (2023). All-cause mortality in moderate and severe covid-19 patients with myocardial injury receiving versus not receiving azvudine: A propensity score-matched analysis. Cardiol. Plus 8, 103–110. doi: 10.1097/CP9.0000000000000049 - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

