Iron transport pathways in the human malaria parasite Plasmodium falciparum revealed by RNA-sequencing
- PMID: 39575308
- PMCID: PMC11578967
- DOI: 10.3389/fcimb.2024.1480076
Iron transport pathways in the human malaria parasite Plasmodium falciparum revealed by RNA-sequencing
Abstract
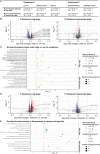
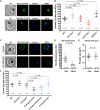
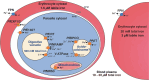
Host iron deficiency is protective against severe malaria as the human malaria parasite Plasmodium falciparum depends on bioavailable iron from its host to proliferate. The essential pathways of iron acquisition, storage, export, and detoxification in the parasite differ from those in humans, as orthologs of the mammalian transferrin receptor, ferritin, or ferroportin, and a functional heme oxygenase are absent in P. falciparum. Thus, the proteins involved in these processes may be excellent targets for therapeutic development, yet remain largely unknown. Here, we show that parasites cultured in erythrocytes from an iron-deficient donor displayed significantly reduced growth rates compared to those grown in red blood cells from healthy controls. Sequencing of parasite RNA revealed diminished expression of genes involved in overall metabolism, hemoglobin digestion, and metabolite transport under low-iron versus control conditions. Supplementation with hepcidin, a specific ferroportin inhibitor, resulted in increased labile iron levels in erythrocytes, enhanced parasite replication, and transcriptional upregulation of genes responsible for merozoite motility and host cell invasion. Through endogenous GFP tagging of differentially expressed putative transporter genes followed by confocal live-cell imaging, proliferation assays with knockout and knockdown lines, and protein structure predictions, we identified six proteins that are likely required for ferrous iron transport in P. falciparum. Of these, we localized PfVIT and PfZIPCO to cytoplasmic vesicles, PfMRS3 to the mitochondrion, and the novel putative iron transporter PfE140 to the plasma membrane for the first time in P. falciparum. PfNRAMP/PfDMT1 and PfCRT were previously reported to efflux Fe2+ from the digestive vacuole. Our data support a new model for parasite iron homeostasis, in which PfE140 is involved in iron uptake across the plasma membrane, PfMRS3 ensures non-redundant Fe2+ supply to the mitochondrion as the main site of iron utilization, PfVIT transports excess iron into cytoplasmic vesicles, and PfZIPCO exports Fe2+ from these organelles in case of iron scarcity. These results provide new insights into the parasite's response to differential iron availability in its environment and into the mechanisms of iron transport in P. falciparum as promising candidate targets for future antimalarial drugs.
Keywords: AlphaFold; Plasmodium falciparum; drug target; gene expression; iron deficiency; malaria; nutrient uptake; transporters.
Copyright © 2024 Wunderlich, Kotov, Votborg-Novél, Ntalla, Geffken, Peine, Portugal and Strauss.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
Identification of a divalent metal transporter required for cellular iron metabolism in malaria parasites.Proc Natl Acad Sci U S A. 2024 Nov 5;121(45):e2411631121. doi: 10.1073/pnas.2411631121. Epub 2024 Oct 28. Proc Natl Acad Sci U S A. 2024. PMID: 39467134 Free PMC article.
-
Characterization of the substrate binding site of an iron detoxifying membrane transporter from Plasmodium falciparum.Malar J. 2021 Jun 30;20(1):295. doi: 10.1186/s12936-021-03827-7. Malar J. 2021. PMID: 34193175 Free PMC article.
-
Recombinant vacuolar iron transporter family homologue PfVIT from human malaria-causing Plasmodium falciparum is a Fe2+/H+exchanger.Sci Rep. 2017 Feb 15;7:42850. doi: 10.1038/srep42850. Sci Rep. 2017. PMID: 28198449 Free PMC article.
-
Vesicle-mediated trafficking of parasite proteins to the host cell cytosol and erythrocyte surface membrane in Plasmodium falciparum infected erythrocytes.Int J Parasitol. 2001 Oct;31(12):1381-91. doi: 10.1016/s0020-7519(01)00256-9. Int J Parasitol. 2001. PMID: 11566305 Review.
-
Protein targeting from malaria parasites to host erythrocytes.Traffic. 2005 Aug;6(8):706-9. doi: 10.1111/j.1600-0854.2005.00310.x. Traffic. 2005. PMID: 15998325 Review.
Cited by
-
Identification of a divalent metal transporter required for cellular iron metabolism in malaria parasites.bioRxiv [Preprint]. 2024 Jun 10:2024.05.10.587216. doi: 10.1101/2024.05.10.587216. bioRxiv. 2024. Update in: Proc Natl Acad Sci U S A. 2024 Nov 5;121(45):e2411631121. doi: 10.1073/pnas.2411631121. PMID: 38798484 Free PMC article. Updated. Preprint.
-
Cell differentiation controls iron assimilation in a choanoflagellate.bioRxiv [Preprint]. 2024 Sep 16:2024.05.25.595918. doi: 10.1101/2024.05.25.595918. bioRxiv. 2024. Update in: mSphere. 2025 Mar 25;10(3):e0091724. doi: 10.1128/msphere.00917-24. PMID: 39345370 Free PMC article. Updated. Preprint.
References
-
- Abrahamian M., Ah-Fong A. M., Davis C., Andreeva K., Judelson H. S. (2016). Gene expression and silencing studies in Phytophthora infestans reveal infection-specific nutrient transporters and a role for the nitrate reductase pathway in plant pathogenesis. PloS Pathog. 12, e1006097. doi: 10.1371/journal.ppat.1006097 - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

