Reducing Dysphagia Following Anterior Cervical Spine Surgery: Insights From a Meta-Analysis
- PMID: 39575355
- PMCID: PMC11579628
- DOI: 10.7759/cureus.74127
Reducing Dysphagia Following Anterior Cervical Spine Surgery: Insights From a Meta-Analysis
Abstract
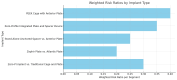
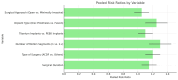
A systematic search was conducted across PubMed, Embase, and Cochrane Library databases to identify relevant studies. The analysis focused on the influence of surgical duration, the number of cervical levels treated, and implant types. A total of 21 studies were included, and heterogeneity among studies was evaluated using the I² statistic. The results indicated that longer surgeries, multi-level procedures, and certain implant designs were associated with an increased risk of dysphagia. In contrast, low-profile implants and stand-alone cage systems demonstrated a reduced risk compared to traditional plate-and-cage constructs. Anterior plates and specific cage designs were linked to higher dysphagia rates. The findings suggest that the risk of dysphagia after anterior cervical spine surgery (ACSS) is influenced by the length of surgery, the number of motion segments treated, and implant design. Optimizing these factors could help reduce postoperative complications and improve patient outcomes.
Keywords: anterior spine surgery; cervical disc disease; post operative dysphagia; spine surgery complications; systematic review and meta analysis.
Copyright © 2024, Ohana et al.
Conflict of interest statement
Conflicts of interest: In compliance with the ICMJE uniform disclosure form, all authors declare the following: Payment/services info: All authors have declared that no financial support was received from any organization for the submitted work. Financial relationships: All authors have declared that they have no financial relationships at present or within the previous three years with any organizations that might have an interest in the submitted work. Other relationships: All authors have declared that there are no other relationships or activities that could appear to have influenced the submitted work.
Figures

References
-
- Adverse events associated with anterior cervical spine surgery. Daniels AH, Riew DK, Yoo J, et al. https://journals.lww.com/jaaos/abstract/2008/12000/adverse_events_associ.... JAAOS. 2008;16:729–738. - PubMed
-
- Are ceramic implants a viable alternative to titanium implants? A systematic literature review. Andreiotelli M, Wenz HJ, Kohal RJ. Clin Oral Implants Res. 2009;20:32–47. - PubMed
-
- Can dysphagia following anterior cervical fusions with rhbmp-2 be reduced with local depomedrol application? A prospective, randomized, placebo-controlled, double-blind trial. Edwards CC 2nd, Dean C, Edwards CC, Phillips D, Blight A. Spine. 2016;41:555–562. - PubMed
-
- Dysphagia after anterior cervical spine surgery: a systematic review of potential preventative measures. Joaquim AF, Murar J, Savage JW, Patel AA. Spine J. 2014;14:2246–2260. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
