Disproportionality analysis of oesophageal toxicity associated with oral bisphosphonates using the FAERS database (2004-2023)
- PMID: 39575385
- PMCID: PMC11578700
- DOI: 10.3389/fphar.2024.1473756
Disproportionality analysis of oesophageal toxicity associated with oral bisphosphonates using the FAERS database (2004-2023)
Abstract
Background: This study analyzed the FDA's Adverse Event Reporting System (FAERS) data to investigate the correlation between oral bisphosphonates (BPs) and oesophageal adverse events (AEs).
Methods: We systematically extracted data on adverse reactions to oral alendronate, risedronate, and ibandronate from the FAERS database, covering the period from the 2004 Q1 to the 2023 Q4. The role_code of AEs mainly includes primary suspect (PS), secondary suspect (SS), concomitant (C), and interaction (I). This study targeted reports with a role_code of "PS." According to the FDA deduplication rule, the latest FDA_DT is selected when the CASEID is the same, and the higher PRIMARYID is selected when the CASEID and FDA_DT are the same. Our analysis leveraged four statistical methods, including the reporting odds ratio (ROR), proportional reporting ratio (PRR), Bayesian confidence propagation neural network (BCPNN), and the multi-item gamma Poisson shrinker (MGPS), to assess the relationship between oral bisphosphonates and oesophageal AEs. The Kaplan-Meier method was utilized to evaluate the cumulative incidence of oesophageal toxicity, while the log-rank test examined the temporal onset profiles of these toxicities. Additionally, the Pearson chi-squared test was employed to identify any significant differences in mortality and hospitalization rates associated with the oesophageal AEs caused by these medications.
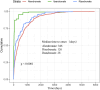
Results: The FAERS database had 41,590 AE reports for oral BPs, with 3,497 (8.41%) related to oesophageal AEs. Our findings indicate that oral BPs are disproportionately associated with an increased incidence of gastrointestinal system AEs at the system organ class (SOC) level. The adverse events identified at the preferred terms (PTs) level encompassed conditions such as gastroesophageal reflux disease, oesophagitis, and oesophageal pain. A significant divergence in the cumulative incidence of oesophageal AEs was observed among patients treated with the three different oral bisphosphonates, as confirmed by the log-rank test (p < 0.0001). Hospitalization rates varied significantly among patients receiving different BPs (p < 0.05), but no significant difference in mortality rates was found.
Conclusion: The study establishes a significant link between oral BPs and oesophageal toxicity, highlighting the need for further research into the mechanisms of BP-induced oesophageal toxicity and potential preventive measures.
Keywords: FAERS; adverse events; bisphosphonates; disproportionality analysis; oesophageal.
Copyright © 2024 Chen, Dai, Song, Zhang and Li.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
Adverse events associated with eteplirsen: A disproportionality analysis using the 2016-2023 FAERS data.Heliyon. 2024 Jun 22;10(13):e33417. doi: 10.1016/j.heliyon.2024.e33417. eCollection 2024 Jul 15. Heliyon. 2024. PMID: 39027557 Free PMC article.
-
A real-world disproportionality analysis of FDA adverse event reporting system (FAERS) events for lecanemab.Front Pharmacol. 2025 Apr 2;16:1559447. doi: 10.3389/fphar.2025.1559447. eCollection 2025. Front Pharmacol. 2025. PMID: 40242445 Free PMC article.
-
Gabapentinoids related psychiatric disorders: an analysis based on the FAERS database from 2004 to 2023.Expert Opin Drug Saf. 2025 Jan 3:1-9. doi: 10.1080/14740338.2024.2448833. Online ahead of print. Expert Opin Drug Saf. 2025. PMID: 39748761
-
Adverse event reporting of faricimab: a disproportionality analysis of FDA adverse event reporting system (FAERS) database.Front Pharmacol. 2025 Mar 12;16:1521358. doi: 10.3389/fphar.2025.1521358. eCollection 2025. Front Pharmacol. 2025. PMID: 40144657 Free PMC article.
-
Post-marketing safety profile of solriamfetol: A real-world disproportionality analysis using FDA adverse event reporting system (FAERS) database.Heliyon. 2024 Sep 25;10(19):e38450. doi: 10.1016/j.heliyon.2024.e38450. eCollection 2024 Oct 15. Heliyon. 2024. PMID: 39391518 Free PMC article.
References
LinkOut - more resources
Full Text Sources

