Inhibition of mitochondrial bioenergetics and hypoxia to radiosensitize diffuse intrinsic pontine glioma
- PMID: 39575457
- PMCID: PMC12083227
- DOI: 10.1093/neuonc/noae255
Inhibition of mitochondrial bioenergetics and hypoxia to radiosensitize diffuse intrinsic pontine glioma
Abstract
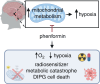
Background: Diffuse intrinsic pontine gliomas (DIPGs) and other H3K27M-mutated diffuse midline gliomas (DMGs) are brain tumors that primarily affect children. Radiotherapy is the standard of care but only provides only temporary symptomatic relief due to radioresistance. Although hypoxia is a major driver of radioresistance in other tumors, there is no definitive evidence that DIPGs are hypoxic. Diffuse intrinsic pontine gliomas often contain histone mutations, which alter tumor metabolism and are also associated with radioresistance. Our objective was to identify the metabolic profiles of DIPG cells, detect hypoxia signatures, and uncover metabolism-linked mechanisms of radioresistance to improve tumor radiosensitivity.
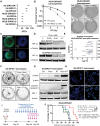
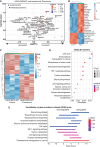
Methods: Using DIPG models combined with clinical datasets, we examined mitochondrial metabolism and signatures of hypoxia. We explored DIPG reliance on mitochondrial metabolism using extracellular flux assays and targeted metabolomics. In vitro and in vivo models were used to explore the mechanisms of targeting mitochondrial bioenergetics and hypoxia for radiosensitization. Treatment-induced transcriptomics and metabolomics were also investigated.
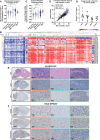
Results: Comprehensive analyses of DIPG cells show signatures of enhanced oxidative phosphorylation (OXPHOS). We also identified increased expression of specific OXPHOS-related genes and signatures of hypoxia gene expression in datasets obtained from DIPG patients. We found the presence of hypoxia in orthotopic mouse models bearing DIPG tumors. These findings enabled us to develop a proof-of-concept treatment strategy to enhance radiosensitivity of DIPGs in vitro and in animal models.
Conclusions: Diffuse intrinsic pontine glioma cells rely on mitochondrial metabolism for growth, and targeting mitochondria disrupts bioenergetics, alleviates hypoxia, and enhances radiosensitivity. These findings warrant further exploration of OXPHOS inhibition as a radiosensitizing strategy for DIPG treatment.
Keywords: diffuse intrinsic pontine gliomas; hypoxia; mitochondria; radiotherapy.
© The Author(s) 2024. Published by Oxford University Press on behalf of the Society for Neuro-Oncology.
Conflict of interest statement
The authors have declared that no conflict of interest exists.
Figures


References
-
- Schwartzentruber J, Korshunov A, Liu XY, et al. Driver mutations in histone H3.3 and chromatin remodelling genes in paediatric glioblastoma. Nature. 2012;482(7384):226–231. - PubMed
-
- Ashton TM, McKenna WG, Kunz-Schughart LA, Higgins GS. Oxidative phosphorylation as an emerging target in cancer therapy. Clin Cancer Res. 2018;24(11):2482–2490. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

