Galectin-1 Elicits a Tissue-Specific Anti-Inflammatory and Anti-Degradative Effect Upon LPS-Induced Response in an Ex Vivo Model of Human Fetal Membranes Modeling an Intraamniotic Inflammation
- PMID: 39575516
- PMCID: PMC11582940
- DOI: 10.1111/aji.70016
Galectin-1 Elicits a Tissue-Specific Anti-Inflammatory and Anti-Degradative Effect Upon LPS-Induced Response in an Ex Vivo Model of Human Fetal Membranes Modeling an Intraamniotic Inflammation
Abstract
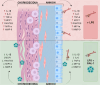
Problem: Intrauterine infection is one of the most jeopardizing conditions associated with adverse outcomes, including preterm birth; however, multiple tolerance mechanisms operate at the maternal-fetal interface to avoid the rejection of the fetus. Among the factors that maintain the uterus as an immunoprivileged site, Galectin-1 (Gal-1), an immunomodulatory glycan-binding protein secreted by the maternal-fetal unit, is pivotal in promoting immune cell homeostasis. This work aimed to evaluate the role of Gal-1 during a lipopolysaccharide (LPS)-induced-inflammatory milieu.
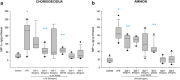
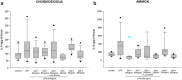
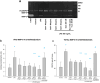
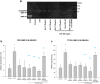
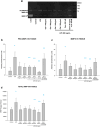
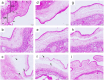
Method of study: Using an ex vivo culture with two independent compartments, human fetal membranes at term were pretreated with 40 and 80 ng/mL of Gal-1, then to reproduce an intraamniotic inflammation, the fetal side of membranes was stimulated with 500 ng/mL of LPS for 24 h. The concentrations of tumor necrosis factor (TNF)-α, interleukin (IL)-1β, IL-6, monocyte chemoattractant protein (MCP1), macrophage inflammatory protein (MIP1) α, regulated upon activation normal T cell expressed and secreted (RANTES), and matrix metalloproteinase (MMP)-9 were measured in both amnion and choriodecidua compartments.
Results: In a tissue-specific fashion profile, pretreatment with the physiologic concentration of Gal-1 significantly diminished the LPS-dependent secretion of TNF-α, IL-1β, Il-6, MCP1, MIP1α, RANTES, and MMP-9.
Conclusion: Gal-1 elicits an anti-inflammatory effect on the human fetal membranes stimulated with LPS, which supports the hypothesis that Gal-1 is part of the immunomodulatory mechanisms intended to stop the harmful effect of inflammation of the maternal-fetal interface.
Keywords: galectin‐1; human chorioamniotic membranes; inflammation; intraamniotic infection; maternal‐fetal unit; preterm birth; preterm labor; tolerogenic.
© 2024 The Author(s). American Journal of Reproductive Immunology published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures





Similar articles
-
Prolactin Protects the Structural Integrity of Human Fetal Membranes by Downregulating Inflammation-induced Secretion of Matrix Metalloproteinases.Immunol Invest. 2022 Jul;51(5):1313-1329. doi: 10.1080/08820139.2021.1936012. Epub 2021 Jun 16. Immunol Invest. 2022. PMID: 34132165
-
Inhibition of PIM1 kinase attenuates inflammation-induced pro-labour mediators in human foetal membranes in vitro.Mol Hum Reprod. 2017 Jun 1;23(6):428-440. doi: 10.1093/molehr/gax013. Mol Hum Reprod. 2017. PMID: 28333279
-
ATF3 is a negative regulator of inflammation in human fetal membranes.Placenta. 2016 Nov;47:63-72. doi: 10.1016/j.placenta.2016.09.006. Epub 2016 Sep 14. Placenta. 2016. PMID: 27780541
-
Activation of AMPK in human fetal membranes alleviates infection-induced expression of pro-inflammatory and pro-labour mediators.Placenta. 2015 Apr;36(4):454-62. doi: 10.1016/j.placenta.2015.01.007. Epub 2015 Jan 26. Placenta. 2015. PMID: 25659498
-
The Effect of Simvastatin on Infection-Induced Inflammatory Response of Human Fetal Membranes.Am J Reprod Immunol. 2015 Jul;74(1):54-61. doi: 10.1111/aji.12372. Epub 2015 Feb 21. Am J Reprod Immunol. 2015. PMID: 25704622
References
-
- Perin J., Mulick A., Yeung D., et al., “Global, Regional, and National Causes of Under‐5 Mortality in 2000–19: An Updated Systematic Analysis With Implications for the Sustainable Development Goals,” Lancet Child Adolesc Health 6, no. 2 (2022): 106–115, 10.1016/S2352-4642(21)00311-4. - DOI - PMC - PubMed
-
- Secretaria de Salud . Cada Año Nacen En México 200 Mil Bebés Prematuros: Secretaría de Salud. 2022. Accessed July 29, 2024. https://www.gob.mx/salud/prensa/558‐cada‐ano‐nacen‐en‐mexico‐200‐mil‐beb....
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

