The Effect of Everolimus Versus Calcineurin Inhibitors on Quality of Life 10-12 Years After Heart Transplantation: The Results of a Randomized Controlled Trial (SCHEDULE Trial)
- PMID: 39575520
- PMCID: PMC11582939
- DOI: 10.1111/ctr.70028
The Effect of Everolimus Versus Calcineurin Inhibitors on Quality of Life 10-12 Years After Heart Transplantation: The Results of a Randomized Controlled Trial (SCHEDULE Trial)
Abstract
Background: Calcineurin inhibitors (CNIs) are associated with long-term complications after heart transplantation (HTx). Everolimus (EVR)-based immunosuppression allows for CNI withdrawal. We used data from The Scandinavian heart transplant everolimus de novo study with early CNI avoidance (SCHEDULE) trial to assess whether health-related quality of life (HRQoL) differed between patients on long-term treatment with EVR versus a CNI-based regimen.
Methods: In SCHEDULE, we randomized 115 patients (mean age 51 ± 13 years, 27% women) to cyclosporine (CNI group; n = 59), or early introduction of EVR and cyclosporine withdrawal within 11 weeks of HTx (EVR group; n = 56). The primary endpoint was the glomerular filtration rate. We used the Short Form-36 (SF-36v2), the EuroQoL visual analogue scale (EQ VAS), and the Beck Depression Inventory (BDI) to assess HRQoL. We re-evaluated the participants after 10-12 years.
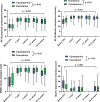
Results: Seventy-eight patients attended follow-up at a median of 11 years after HTx. The SF-36 physical component summary score increased from 32 ± 10 pre-HTx to 44 ± 12 11 years after HTx (p < 0.01) in the EVR group and from 33 ± 9 to 44 ± 11 (p < 0.01) with CNI. The mental component summary score increased from 46 ± 12 to 53 ± 13 (EVR); p = 0.04 and from 38 ± 13 to 49 ± 13 (CNI); p < 0.01. Similar improvements were observed regarding EQ-VAS and the BDI. There were no significant between-group differences for either measure of HRQoL.
Conclusions: In heart transplant recipients, an EVR-based immunosuppressive strategy resulted in similar long-term improvements in HRQoL as treatment with a CNI-based regimen.
Keywords: calcineurin inhibitors; cyclosporine; everolimus; health‐related quality of life; heart transplantation; immunosuppression; quality of life.
© 2024 The Author(s). Clinical Transplantation published by Wiley Periodicals LLC.
Conflict of interest statement
This is a long‐term follow‐up study of the randomized SCHEDULE‐trial performed in collaboration with Novartis where Novartis provided some financial support. Finn Gustafsson receives advisor fees from Abbott, Bayer, Carmat, Impulse Dynamics, Novartis, and Pfizer and speaker fees from AstraZeneca, Boehringer Ingelheim, and Orion Pharma. Kaspar Broch has received lecture fees from Amgen, AstraZeneca, Bayer, Boehringer, Novartis, Novo Nordisk, Pfizer, Pharmacosmos, and Vifor Pharma. Lars Gullestad has received lecture fees from Novartis, Boehringer Ingelheim, and AstraZeneca. Einar Gude has received lecture fees from Pfizer, Merck Sharp and Dohme, and Novartis. Niklas Bergh is currently employed as Senior Medical Director at AstraZeneca, Mölndal, Sweden. Thea Halden is an employee at Novartis Norge AS. The other authors declare no conflicts of interest.
Figures
Similar articles
-
Effect of everolimus vs calcineurin inhibitors on quality of life in heart transplant recipients during a 3-year follow-up: Results of a randomized controlled trial (SCHEDULE).Clin Transplant. 2017 Sep;31(9). doi: 10.1111/ctr.13038. Epub 2017 Jul 19. Clin Transplant. 2017. PMID: 28640529 Clinical Trial.
-
Long-term follow-up of the randomized, prospective Scandinavian heart transplant everolimus de novo study with early calcineurin inhibitors avoidance (SCHEDULE) trial.J Heart Lung Transplant. 2024 Dec;43(12):1948-1959. doi: 10.1016/j.healun.2024.07.002. Epub 2024 Jul 20. J Heart Lung Transplant. 2024. PMID: 39038562 Clinical Trial.
-
Effect of Calcineurin Inhibitor-Free, Everolimus-Based Immunosuppressive Regimen on Albuminuria and Glomerular Filtration Rate After Heart Transplantation.Transplantation. 2017 Nov;101(11):2793-2800. doi: 10.1097/TP.0000000000001706. Transplantation. 2017. PMID: 28230646 Clinical Trial.
-
Everolimus with early withdrawal or reduced-dose calcineurin inhibitors improves renal function in liver transplant recipients: A systematic review and meta-analysis.Clin Transplant. 2017 Feb;31(2). doi: 10.1111/ctr.12872. Epub 2017 Jan 18. Clin Transplant. 2017. PMID: 27862340
-
Long-Term Outcomes of Everolimus Therapy in De Novo Liver Transplantation: A Systematic Review and Meta-Analysis of Randomized Controlled Trials.Transplant Proc. 2021 Jan-Feb;53(1):148-158. doi: 10.1016/j.transproceed.2020.09.021. Epub 2021 Jan 1. Transplant Proc. 2021. PMID: 33390288
Cited by
-
Innovative Therapeutic Strategies for Myocardial Infarction Across Various Stages: Non-Coding RNA and Stem Cells.Int J Mol Sci. 2024 Dec 30;26(1):231. doi: 10.3390/ijms26010231. Int J Mol Sci. 2024. PMID: 39796085 Free PMC article. Review.
References
-
- Dellgren G., Westerlind A., Liden H., et al., “Continuous Improvement in Outcome After Heart Transplantation—Long‐Term Follow‐Up After Three Decades of Experience,” International Journal of Cardiology 231 (2017): 188–194. - PubMed
-
- Velleca A., Shullo M. A., Dhital K., et al., “The International Society for Heart and Lung Transplantation (ISHLT) Guidelines for the Care of Heart Transplant Recipients,” Journal of Heart and Lung Transplantation 42 (2023): e1–e141. - PubMed
-
- Khush K. K., Hsich E., Potena L., et al., “The International Thoracic Organ Transplant Registry of the International Society for Heart and Lung Transplantation: Thirty‐Eighth Adult Heart Transplantation Report – 2021; Focus on Recipient Characteristics,” Journal of Heart and Lung Transplantation 40 (2021): 1035–1049. - PMC - PubMed
-
- Tackmann E. and Dettmer S., “Health‐Related Quality of Life in Adult Heart‐Transplant Recipients – A Systematic Review,” Herz 45 (2020): 475–482. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical