AMPK associates with and causes fragmentation of the Golgi by phosphorylating the guanine nucleotide exchange factor GBF1
- PMID: 39575556
- PMCID: PMC11827860
- DOI: 10.1242/jcs.262182
AMPK associates with and causes fragmentation of the Golgi by phosphorylating the guanine nucleotide exchange factor GBF1
Abstract
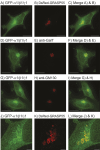
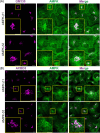
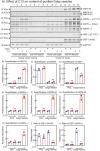
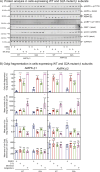
AMP-activated protein kinase (AMPK) is an energy sensor that regulates cellular functions in response to changes in energy availability. However, whether AMPK activity is spatially regulated, and the implications for cell function, have been unclear. We now report that AMPK associates with the Golgi, and that its activation by two specific pharmacological activators leads to Golgi fragmentation similar to that caused by the antibiotic Golgicide A, an inhibitor of Golgi-specific Brefeldin A resistance factor-1 (GBF1), a guanine nucleotide exchange factor that targets ADP-ribosylation factor 1 (ARF1). Golgi fragmentation in response to AMPK activators is lost in cells carrying gene knockouts of AMPK-α subunits. AMPK has been previously reported to phosphorylate GBF1 at residue Thr1337, and its activation causes phosphorylation at that residue. Importantly, Golgi disassembly upon AMPK activation is blocked in cells expressing a non-phosphorylatable GBF1-T1337A mutant generated by gene editing. Furthermore, the trafficking of a plasma membrane-targeted protein through the Golgi complex is delayed by AMPK activation. Our findings provide a mechanism to link AMPK activation during cellular energy stress to downregulation of protein trafficking involving the Golgi.
Keywords: AMP-activated protein kinase; Golgi; Membrane trafficking; Protein secretion; Subcellular localization.
© 2024. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interests The authors declare no competing or financial interests.
Figures



References
-
- Baillie, G. S., Huston, E., Scotland, G., Hodgkin, M., Gall, I., Peden, A. H., Mackenzie, C., Houslay, E. S., Currie, R., Pettitt, T. R.et al. (2002). TAPAS-1, a novel microdomain within the unique N-terminal region of the PDE4A1 cAMP-specific phosphodiesterase that allows rapid, Ca2+-triggered membrane association with selectivity for interaction with phosphatidic acid. J. Biol. Chem. 277, 28298-28309. 10.1074/jbc.M108353200 - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

