Pharmacokinetic Modeling and Model-Based Hypothesis Generation for Dose Optimization of Clonidine in Neonates With Neonatal Opioid Withdrawal Syndrome
- PMID: 39575611
- PMCID: PMC11993295
- DOI: 10.1002/cpt.3507
Pharmacokinetic Modeling and Model-Based Hypothesis Generation for Dose Optimization of Clonidine in Neonates With Neonatal Opioid Withdrawal Syndrome
Abstract
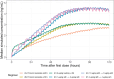
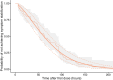
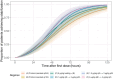
The No-POPPY study (NCT03396588), a double-blind, randomized trial compared morphine with clonidine therapy for neonatal opioid withdrawal syndrome (NOWS) and found that the duration of treatment was similar across groups. This is significant because perinatal use of morphine has the potential for neurodevelopmental consequences. Still, the clonidine group reached symptom stabilization (Finnegan score (FS) < 8) later than the morphine group and had a more significant number of patients who required adjunct therapy. However, the mean FS was consistently lower in the clonidine group after day 6. This prompted us to use pharmacokinetic (PK) and parametric time-to-event (TTE) modeling to simulate dosage schedules that may decrease the time to stabilization and reduce the need for adjunct therapy. Population PK (popPK) analysis was conducted, and the final model was a one-compartment model with first-order absorption and elimination, incorporating allometric scaling and age effect on apparent clearance (CL/F) and apparent volume (V/F). The population estimates for CL/F and V/F were 13.6 L/h/70 kg and 416 L/70 kg, respectively, similar to the reported values. A Weibull model described the TTE data best, followed by incorporating predicted average concentrations to yield the final Weibull accelerated failure time model. Simulations of dosing strategies showed that increasing both the starting and maximum doses could potentially shorten the time to stabilization, and thus, length of treatment and hospital stay. Given the hypothesis-generating nature of this analysis, the recommended dosing regimens should be tested prospectively to evaluate their benefits.
© 2024 The Author(s). Clinical Pharmacology & Therapeutics published by Wiley Periodicals LLC on behalf of American Society for Clinical Pharmacology and Therapeutics.
Conflict of interest statement
FT was an employee of the University of Kentucky when the work was initially conducted and is now an employee of Genentech, Inc. CMN was an employee of the University of Kentucky when the work was initially conducted and is now an employee of NewGround Pharmaceutical Consulting LLC. All other authors declared no competing interests in this work. Genentech, Inc. and NewGround Pharmaceutical Consulting LLC do not fund this study and have no roles in its design or conduct.
Figures

References
-
- Hudak, M.L. & Tan, R.C. Neonatal drug withdrawal. Pediatrics 129, e540–e560 (2012). - PubMed
-
- Patrick, S.W. , Schumacher, R.E. , Benneyworth, B.D. , Krans, E.E. , McAllister, J.M. & Davis, M.M. Neonatal abstinence syndrome and associated health care expenditures: United States, 2000‐2009. JAMA 307, 1934–1940 (2012). - PubMed
-
- Davidson, A. & Flick, R.P. Neurodevelopmental implications of the use of sedation and analgesia in neonates. Clin. Perinatol. 40, 559–573 (2013). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

