Cytokine Adsorption During Ex Vivo Blood Perfusion Improves Contractility of Donation After Circulatory Death Hearts
- PMID: 39575707
- PMCID: PMC11681589
- DOI: 10.1161/JAHA.124.036872
Cytokine Adsorption During Ex Vivo Blood Perfusion Improves Contractility of Donation After Circulatory Death Hearts
Abstract
Background: Donation after circulatory death (DCD) hearts have to withstand ischemia/reperfusion injury that is partially driven by proinflammatory cytokines and decreases ventricular contractility. We hypothesize that cytokine adsorption during normothermic ex vivo blood perfusion of DCD hearts reduces the cytokine levels and improves ventricular contractility.
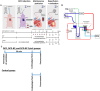
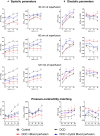
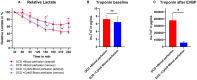
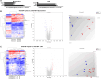
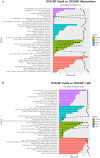
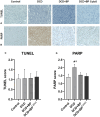
Methods and results: Porcine DCD hearts were maintained 4 hours by ex vivo blood perfusion with (DCD-BPCytoS, all groups: N=8) or without (DCD-BP) CytoSorb, followed by 2 hours reperfusion with fresh blood, including left ventricular functional analysis using a balloon catheter. In a control and a DCD group, hearts were evaluated after procurement. We determined lactate and cytokines after ex vivo blood perfusion and the myocardial and left anterior descending artery transcriptome using microarrays after reperfusion. In DCD-BPCytoS, the developed pressure (control: 124±7 mm Hg/s, DCD: 86±4 mm Hg/s, DCD-BP: 69±11 mm Hg/s, DCD-BPCytoS: 112±9 mm Hg/s; P<0.05) and maximal slope of pressure increment (control: 2010±39 mm Hg/s, DCD: 1219±164 mm Hg/s, DCD-BP: 964±163 mm Hg/s, DCD-BPCytoS: 1794±205 mm Hg/s; P<0.05) were higher compared with DCD-BP and DCD hearts. However, contractility decreased later during reperfusion without CytoSorb. After 4 hours, troponin, lactate (45±5% versus 69±9%, P<0.05), IL (interleukin)-1β, -1ra, and -8 were lower in DCD-BPCytoS hearts. In the myocardium of DCD-BPCytoS compared with DCD-BP hearts, inflammatory mediator receptor activity/binding pathways were enriched, and pathways for collagen-containing extracellular matrix and contractile fiber were underrepresented. In the left anterior descending artery of DCD-BPCytoS hearts, serine/threonine/tyrosine kinase activity and wound-healing pathways were enriched, and mitochondrial protein-containing complex and respiratome-associated pathways were underrepresented.
Conclusions: CytoSorb during ex vivo blood perfusion enhances the maintenance of DCD hearts and is likely to improve graft function after transplantation.
Keywords: CytoSorb; cytokine adsorption; cytokines; donation after circulatory death; heart transplantation; machine perfusion.
Figures


References
-
- Messer S, Cernic S, Page A, Berman M, Kaul P, Colah S, Ali J, Pavlushkov E, Baxter J, Quigley R, et al. A 5‐year single‐center early experience of heart transplantation from donation after circulatory‐determined death donors. J Heart Lung Transplant. 2020;39:1463–1475. doi: 10.1016/j.healun.2020.10.001 - DOI - PubMed
-
- Miñambres E, Royo‐Villanova M, Pérez‐Redondo M, Coll E, Villar‐García S, Canovas SJ, Francisco Nistal J, Garrido IP, Gómez‐Bueno M, Cobo M, et al. Spanish experience with heart transplants from controlled donation after the circulatory determination of death using thoraco‐abdominal normothermic regional perfusion and cold storage. Am J Transplant. 2021;21:1597–1602. doi: 10.1111/ajt.16446 - DOI - PubMed
-
- Saemann L, Korkmaz‐Icöz S, Hoorn F, Veres G, Kraft P, Georgevici A‐I, Brune M, Guo Y, Loganathan S, Wenzel F, et al. Reconditioning of circulatory death hearts by ex‐vivo machine perfusion with a novel HTK‐N preservation solution. J Heart Lung Transplant. 2021;40:1135–1144. doi: 10.1016/j.healun.2021.07.009 - DOI - PubMed
-
- Moeslund N, Ertugrul IA, Hu MA, Dalsgaard FF, Ilkjaer LB, Ryhammer P, Pedersen M, Erasmus ME, Eiskjaer H. Ex‐situ oxygenated hypothermic machine perfusion in donation after circulatory death heart transplantation following either direct procurement or in‐situ normothermic regional perfusion. J Heart Lung Transplant. 2023;42:730–740. doi: 10.1016/j.healun.2023.01.014 - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

