Hereditary Transthyretin Amyloidosis in Patients Referred to a Genetic Testing Program
- PMID: 39575713
- PMCID: PMC11681574
- DOI: 10.1161/JAHA.123.033770
Hereditary Transthyretin Amyloidosis in Patients Referred to a Genetic Testing Program
Abstract
Background: Diagnosis of hereditary amyloid transthyretin (hATTR) amyloidosis with cardiomyopathy is frequently delayed, in large part because of symptom overlap with other cardiovascular diseases and limited provider knowledge of this disease. The sponsored and provider referred hATTR Compass Genetic Testing Program (Ionis, Carlsbad, CA; Ambry Genetics, Aliso Viejo, CA) provided no-cost genetic testing to adults with a family history or clinical suspicion of hATTR amyloidosis. This study aims to characterize patients with hATTR amyloidosis and increase awareness of genetic testing for hATTR.
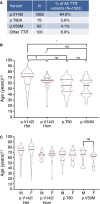
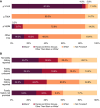
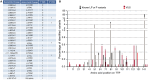
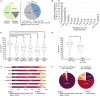
Methods and results: Patients were referred to the hATTR genetic testing program, and a cross-sectional post hoc analysis was performed. A pathogenic TTR variant was identified in 1503 (6.6%) of 22 886 patients referred for genetic testing between June 2018 and March 2022. Patients were identified in all US states, 3 US territories, and Canada. Median age at testing was 63 years, and 44% were female. The p.V142I TTR variant was the most common (n=1263, 84.0%). Only 32% of patients with a pathogenic TTR variant reported a known family history; a lower percentage of Black individuals reported a known family history compared with other racial and ethnic groups. Black patients accounted for 23.7% of all patients referred and 81.9% of patients with the p.V142I variant.
Conclusions: This sponsored genetic testing program identified a large number of patients with a pathogenic TTR variant, notably, in geographic regions not previously reported, and demographic groups that are historically underrepresented in the literature.
Keywords: amyloidosis; cardiomyopathy; genetic testing; prevalence; transthyretin.
Figures

Comment in
-
Improving Health Equity Through Standardization and Selective Expansion of Genetic Testing in Transthyretin Amyloidosis.J Am Heart Assoc. 2024 Dec 3;13(23):e036995. doi: 10.1161/JAHA.124.036995. Epub 2024 Nov 22. J Am Heart Assoc. 2024. PMID: 39575714 Free PMC article. No abstract available.
References
-
- Gertz M, Adams D, Ando Y, Beirão JM, Bokhari S, Coelho T, Comenzo RL, Damy T, Dorbala S, Drachman BM, et al. Avoiding misdiagnosis: expert consensus recommendations for the suspicion and diagnosis of transthyretin amyloidosis for the general practitioner. BMC Fam Pract. 2020;21:198. doi: 10.1186/s12875-020-01252-4 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

