Mortality Risk in Patients With Cardiac Complications Following Ischemic Stroke: A Report From the Virtual International Stroke Trials Archive
- PMID: 39575723
- PMCID: PMC11681577
- DOI: 10.1161/JAHA.124.036799
Mortality Risk in Patients With Cardiac Complications Following Ischemic Stroke: A Report From the Virtual International Stroke Trials Archive
Abstract
Background: Cardiac complications may occur in patients following ischemic stroke (stroke-heart syndrome [SHS]). We investigated the mortality risk in patients with SHS and across the SHS manifestations.
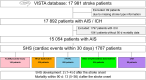
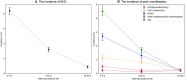
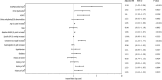
Methods and results: Data were sought from the VISTA (Virtual International Stroke Trials Archive), an international repository of clinical trials data. We reviewed relevant adverse events and classified patients into 2 cohorts based on the incidence of SHS. The SHS was defined as developing any cardiac complications within 30 days following stroke. Using Cox proportional hazards models, we evaluated the temporal risk dynamics of 90-day death associated with the day of SHS onset. We also compared the risk of 90-day death across SHS manifestations, using multivariate analysis. Among 15 054 patients with ischemic stroke (mean age, 69±12 years; 55% men), 1787 (11.8% [95% CI, 11.3-12.3]) developed SHS. The median onset time for SHS was 2 (interquartile range, 1-4) days. The most prevalent manifestation was other arrhythmia/ECG abnormalities, with the incidence rate of 6.5% (95% CI, 6.1-6.9). Patients who developed SHS between 10 and 30 days following stroke had significantly higher risks of death compared with those with SHS within the first 0 to 3 days (adjusted hazard ratio, 1.84 [95% CI, 1.36-2.49]). In the multivariate-adjusted analysis, SHS manifested as acute myocardial injury/myocardial injury, heart failure/left ventricular dysfunction, and atrial fibrillation/flutter were associated with the highest risk of death within 90 days after stroke across SHS manifestations excluding cardiorespiratory arrest.
Conclusions: SHS is associated with a high risk of death, with a greater risk observed with delayed SHS onset.
Keywords: death; ischemic stroke; stroke‐heart syndrome.
Conflict of interest statement
Dr Dawson has received speaker fees from AstraZeneca, Bayer, Boehringer Ingelheim, Bristol‐Myers Squibb, Daiichi Sankyo, Medtronic, and Pfizer, as well as a travel fee from MicroTransponder Inc. He also received an investigator‐initiated research funding grant from the Stroke Association UK. Dr Lip reports consultant and speaker for BMS/Pfizer, Boehringer Ingelheim, Daiichi‐Sankyo, and Anthos. No fees are received personally. He is a National Institute for Health and Care Research senior investigator and co‐principal investigator of the Atrial Fibrillation Integrated Approach in Frail, Multimorbid, and Pollymedicated Older People project on multimorbidity in AF (grant agreement No. 899871), TARGET project on digital twins for personalized management of atrial fibrillation and stroke (grant agreement No. 101136244) and Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation (ARISTOTLE) project on artificial intelligence for management of chronic long term conditions (grant agreement No 101080189), which are all funded by the European Union's Horizon Europe Research and Innovation program. The remaining authors have no disclosures to report.
Figures

References
-
- Feigin VL, Forouzanfar MH, Krishnamurthi R, Mensah GA, Connor M, Bennett DA, Moran AE, Sacco RL, Anderson L, Truelsen T, et al. Global and regional burden of stroke during 1990–2010: findings from the global burden of disease study 2010. Lancet. 2014;383:245–255. doi: 10.1016/S0140-6736(13)61953-4 - DOI - PMC - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials

