Prospective Observational Cohort Study of Tenecteplase: Results From the Indian Registry in Ischemic Stroke-Tenecteplase
- PMID: 39575724
- PMCID: PMC11681559
- DOI: 10.1161/JAHA.124.036382
Prospective Observational Cohort Study of Tenecteplase: Results From the Indian Registry in Ischemic Stroke-Tenecteplase
Abstract
Background: Tenecteplase has been approved for acute ischemic stroke at a dose of 0.2 mg/kg by the Indian licensing authority. A registry to evaluate the safety of tenecteplase was mandated by the licensing authority. The research aim was to use the Indian Registry in Ischemic Stroke-Tenecteplase (IRIS-TNK) to assess the safety and clinical outcomes in patients treated with tenecteplase in routine clinical practice.
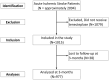
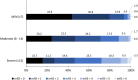
Methods and results: In this prospective, registry-based observational, cohort study, the primary outcome was proportion of symptomatic intracerebral hemorrhages at 36±6 hours after treatment. Secondary outcomes included improvement in National Institutes of Health Stroke Scale (NIHSS) score by either ≥4 or 8 points or an NIHSS score of 0, assessment of excellent outcome (modified Rankin Scale score 0 or 1), functional independence (modified Rankin Scale score 0-2), and Barthel Index score. From October 2017 to May 2023, 1015 patients with a median age of 62 years (interquartile range [IQR, 52-71 years]) were recruited across India. The median baseline NIHSS score was 9 (IQR, 6-13). The proportion of patients with symptomatic intracerebral hemorrhage was 0.6% (95% CI, 0.2-1.3%), and 10 patients (1% [95% CI, 0.5-1.9%]) died within 3 months. Improvement in NIHSS score by ≥4 points or an NIHSS score of 0 at 24 hours was observed in 34.4% (95% CI, 31.5-37.4%) of patients. An excellent outcome (modified Rankin Scale score 0 or 1) at 3 months was achieved in 55.4% (95% CI, 52.3-58.5%) of patients.
Conclusions: These results confirm that tenecteplase at a dose of 0.2 mg/kg is safe in routine clinical practice, when administered within 4.5 hours of symptom onset.
Registration: https://ctri.nic.in/Clinicaltrials/. Identifier: CTRI/2017/11/010380.
Keywords: acute ischemic stroke; hemorrhages; standard of care; tenecteplase; thrombolytic therapy.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

