Plasma pTau217 ratio predicts continuous regional brain tau accumulation in amyloid-positive early Alzheimer's disease
- PMID: 39575854
- PMCID: PMC11848419
- DOI: 10.1002/alz.14411
Plasma pTau217 ratio predicts continuous regional brain tau accumulation in amyloid-positive early Alzheimer's disease
Abstract
Background: This study examines whether phosphorylated plasma Tau217 ratio (pTau217R) can predict tau accumulation in different brain regions, as measured by positron emission tomography (PET) standardized uptake value ratio (SUVR), for staging Alzheimer's disease (AD).
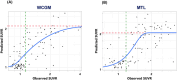
Methods: Plasma pTau217R was measured using immunoprecipitation-mass spectrometry. Models for predicting tau PET SUVR, developed with 144 early AD individuals using [18F]MK6240, were validated in two validation sets, VS1 (98 early AD) and VS2 (47 preclinical/early AD with a different tracer, flortaucipir (Tauvid)), all amyloid-beta positive (Aβ+).
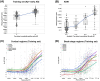
Results: The pTau217R-based model predicted tau levels up to an SUVR of 2 in multiple brain regions, effectively assessing tau status at different tau levels with receiver operating characteristic (ROC) curve areas of 0.84-0.95 in VS1 and 0.71-0.88 in VS2 (using a different tracer). It reduced PET scan needs by 65% while maintaining 95% sensitivity.
Discussion: PTau217R reliably predicts regional tau accumulation in early AD, reducing reliance on tau PET scans and broadening its clinical application.
Clinical trial registration number: NCT03887455 (ClarityAD) HIGHLIGHTS: Developed a model using plasma pTau217R to predict tau levels across brain regions. pTau217R model outperformed models based on clinical, MRI, and other blood biomarkers. The model reliably predicted tau levels exceeding tau positivity and higher thresholds. Screening with pTau217R could reduce tau PET scans by 65% at 95% sensitivity. pTau217R model aids in disease staging and monitoring in early AD.
Keywords: Tau PET; blood–based biomarkers; diagnosis; disease continuum; disease staging; patient monitoring; patient screening; prediction.
© 2024 Eisai Inc. Alzheimer's & Dementia published by Wiley Periodicals LLC on behalf of Alzheimer's Association.
Conflict of interest statement
V.D., A.C., K.H., T.D., E.A., Y.Y., P.S., L.K.M., J.Z., L.R., H.H., L.D.K., S.D., and M.C.I. are employees of Eisai. K.H. is an Eisai‐sponsored voluntary research associate professor at Washington University and receives a salary from Eisai. H.H. is a Reviewing Editor and previously a Senior Associate Editor for the journal Alzheimer's & Dementia, and he was not involved in the editorial process. No competing disclosures to report for D.A.L. Author disclosures are available in the Supporting Information.
Figures

References
-
- 2023 Alzheimer's disease facts and figures. Alzheimers Dement. 2023;19:1598‐1695. - PubMed
-
- Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science. 2002;297:353‐356. - PubMed
-
- Braak H, Braak E. Neuropathological stageing of Alzheimer‐related changes. Acta Neuropathol. 1991;82:239‐259. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

