Pharmacokinetics, toxicities, and tissue concentrations of belotecan sprayed by rotational intraperitoneal pressurized aerosol chemotherapy in a pig model
- PMID: 39575997
- PMCID: PMC12099050
- DOI: 10.3802/jgo.2025.36.e37
Pharmacokinetics, toxicities, and tissue concentrations of belotecan sprayed by rotational intraperitoneal pressurized aerosol chemotherapy in a pig model
Abstract
Objective: We evaluated the pharmacokinetics, tissue concentrations, and toxicities of belotecan during rotational intraperitoneal pressurized aerosol chemotherapy (RIPAC) in pigs.
Methods: We sprayed belotecan in 10% and 30% of doses for intravenous chemotherapy in six pigs (cohort 1, n=3, 0.50 mg/m²; cohort 2, n=3, 1.5 mg/m²). We evaluated the time-dependent plasma concentrations of belotecan before RIPAC to 120 hours for the pharmacokinetics, tissue concentrations in twelve peritoneal regions, and hepatic and renal functions before RIPAC to 120 hours in the 2 cohorts.
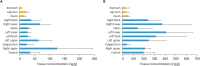
Results: Mean values of the peak plasma concentration (Cmax), the time to Cmax, the time taken for Cmax to drop in half, and the area under the curve from time zero to the time of last quantifiable concentration were 905 and 3,700 ng/mL, 1.42 and 1.50 hours, 3.64 and 5.60 hours, and 2,260 and 17,900 pg·hr/mL in cohorts 1 and 2, respectively. Mean values of tissue concentrations were 1.5 to 15.3 times higher in cohort 1 than in cohort 2 despite the similar ratio of tissue to plasma concentration, and tissue concentrations in the two cohorts were higher in the parietal peritoneum than in the visceral peritoneum. However, hepatic and renal functions were not different before RIPAC to 120 hours in the two cohorts.
Conclusion: RIPAC using belotecan of 0.5 mg/m² and 1.5 mg/m² may be feasible with fewer hepatic and renal toxicities in pigs. Thus, belotecan of 1.5 mg/m² may be considered as the starting dose for RIPAC in a phase 1 trial.
Keywords: Aerosols; Drug Therapy; Pharmacokinetics; Swine; Tissues.
© 2025. Asian Society of Gynecologic Oncology, Korean Society of Gynecologic Oncology, and Japan Society of Gynecologic Oncology.
Conflict of interest statement
Hee Seung Kim is the Chief Executive Officer at Dreampac Corp. (Wonju, Republic of Korea). Moreover, Seungmee Lee and San-Hui Lee are the Chief Executive Officer and a director of Precision Medicine for Peritoneal Metastasis Corp. (Wonju, Republic of Korea). The other authors have no conflict of interest.
Figures

Similar articles
-
Rotational intraperitoneal pressurized aerosol chemotherapy with paclitaxel and cisplatin: pharmacokinetics, tissue concentrations, and toxicities in a pig model.J Gynecol Oncol. 2022 Sep;33(5):e56. doi: 10.3802/jgo.2022.33.e56. Epub 2022 May 24. J Gynecol Oncol. 2022. PMID: 35712969 Free PMC article.
-
Development of rotational intraperitoneal pressurized aerosol chemotherapy to enhance drug delivery into the peritoneum.Drug Deliv. 2021 Dec;28(1):1179-1187. doi: 10.1080/10717544.2021.1937382. Drug Deliv. 2021. PMID: 34121568 Free PMC article.
-
Optimal Nozzle Position and Patient's Posture to Enhance Drug Delivery into the Peritoneum during Rotational Intraperitoneal Pressurized Aerosol Chemotherapy in a Swine Model.J Pers Med. 2022 Oct 31;12(11):1799. doi: 10.3390/jpm12111799. J Pers Med. 2022. PMID: 36579527 Free PMC article.
-
Tiotropium bromide. A review of its use as maintenance therapy in patients with COPD.Treat Respir Med. 2004;3(4):247-68. doi: 10.2165/00151829-200403040-00005. Treat Respir Med. 2004. PMID: 15350163 Review.
-
Mitoxantrone: a review of its use in multiple sclerosis.CNS Drugs. 2004;18(6):379-96. doi: 10.2165/00023210-200418060-00010. CNS Drugs. 2004. PMID: 15089110 Review.
References
-
- Mirza MR, Monk BJ, Herrstedt J, Oza AM, Mahner S, Redondo A, et al. Niraparib maintenance therapy in platinum-sensitive, recurrent ovarian cancer. N Engl J Med. 2016;375:2154–2164. - PubMed
-
- Aghajanian C, Blank SV, Goff BA, Judson PL, Teneriello MG, Husain A, et al. OCEANS: a randomized, double-blind, placebo-controlled phase III trial of chemotherapy with or without bevacizumab in patients with platinum-sensitive recurrent epithelial ovarian, primary peritoneal, or fallopian tube cancer. J Clin Oncol. 2012;30:2039–2045. - PMC - PubMed
-
- Shi T, Zhu J, Feng Y, Tu D, Zhang Y, Zhang P, et al. Secondary cytoreduction followed by chemotherapy versus chemotherapy alone in platinum-sensitive relapsed ovarian cancer (SOC-1): a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2021;22:439–449. - PubMed
-
- Harter P, Sehouli J, Vergote I, Ferron G, Reuss A, Meier W, et al. Randomized trial of cytoreductive surgery for relapsed ovarian cancer. N Engl J Med. 2021;385:2123–2131. - PubMed
-
- Park SJ, Lee EJ, Lee TS, Wang KL, Okamoto A, Ochiai K, et al. Asian perspective on debulking surgery for advanced ovarian cancer: an E-survey. Eur J Surg Oncol. 2021;47:1111–1116. - PubMed
MeSH terms
Substances
Grants and funding
- 2710019525/MSIT/Ministry of Science and ICT/Korea
- RS-2024-00441788/Accelerator Investment-Driven Tech Incubator Program for Startup/Korea
- 0620211700/SNUH/Seoul National University Hospital/Korea
- 0320232140/SNUH/Seoul National University Hospital/Korea
- 0320212060/SNUH/Seoul National University Hospital/Korea

