Comparative Physiology and Morphology of BLA-Projecting NBM/SI Cholinergic Neurons in Mouse and Macaque
- PMID: 39576005
- PMCID: PMC11583843
- DOI: 10.1002/cne.70001
Comparative Physiology and Morphology of BLA-Projecting NBM/SI Cholinergic Neurons in Mouse and Macaque
Abstract
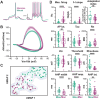
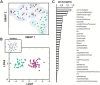
Cholinergic projection neurons of the nucleus basalis and substantia innominata (NBM/SI) densely innervate the basolateral amygdala (BLA) and have been shown to contribute to the encoding of fundamental and life-threatening experiences. Given the vital importance of these circuits in the acquisition and retention of memories that are essential for survival in a changing environment, it is not surprising that the basic anatomical organization of the NBM/SI is well conserved across animal classes as diverse as teleost and mammal. What is not known is the extent to which the physiology and morphology of NBM/SI neurons have also been conserved. To address this issue, we made patch-clamp recordings from NBM/SI neurons in ex vivo slices of two widely divergent mammalian species, mouse and rhesus macaque, focusing our efforts on cholinergic neurons that project to the BLA. We then reconstructed most of these recorded neurons post hoc to characterize neuronal morphology. We found that rhesus macaque BLA-projecting cholinergic neurons were both more intrinsically excitable and less morphologically compact than their mouse homologs. Combining measurements of 18 physiological features and 13 morphological features, we illustrate the extent of the separation. Although macaque and mouse neurons both exhibited considerable within-group diversity and overlapped with each other on multiple individual metrics, a combined morphoelectric analysis demonstrates that they form two distinct neuronal classes. Given the shared purpose of the circuits in which these neurons participate, this finding raises questions about (and offers constraints on) how these distinct classes result in similar behavior.
Keywords: basal forebrain; cholinergic; donkey anti‐goat Alexa‐Fluor 488; morphoelectric physiology; mouse; nonhuman primate.
Published 2024. This article is a U.S. Government work and is in the public domain in the USA. The Journal of Comparative Neurology published by Wiley Periodicals LLC.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
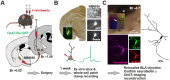
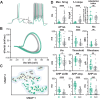

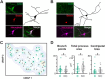
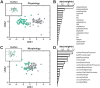



Update of
-
Comparative physiology and morphology of BLA-projecting NBM/SI cholinergic neurons in mouse and macaque.Res Sq [Preprint]. 2024 Aug 2:rs.3.rs-4824445. doi: 10.21203/rs.3.rs-4824445/v1. Res Sq. 2024. Update in: J Comp Neurol. 2024 Nov;532(11):e70001. doi: 10.1002/cne.70001. PMID: 39149491 Free PMC article. Updated. Preprint.
References
-
- Alonso, A. , Khateb A., Fort P., Jones B. E., and Mühlethaler M.. 1996. “Differential Oscillatory Properties of Cholinergic and Non‐Cholinergic Nucleus Basalis Neurons in Guinea Pig Brain Slice.” European Journal of Neuroscience 8: 169–182. - PubMed
-
- Bengtson, C. P. , and Osborne P. B.. 2000. “Electrophysiological Properties of Cholinergic and Noncholinergic Neurons in the Ventral Pallidal Region of the Nucleus Basalis in Rat Brain Slices.” Journal of Neurophysiology 83: 2649–2660. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

