Cellular signatures in human blood track bone mineral density in postmenopausal women
- PMID: 39576015
- PMCID: PMC11601907
- DOI: 10.1172/jci.insight.178977
Cellular signatures in human blood track bone mineral density in postmenopausal women
Abstract
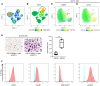
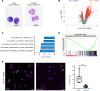
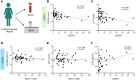
Osteoclasts are the sole bone-resorbing cells and are formed by the fusion of osteoclast precursor cells (OCPs) derived from myeloid lineage cells. Animal studies reveal that circulating OCPs (cOCPs) in blood travel to bone and fuse with bone-resident osteoclasts. However, the characteristics of human cOCPs and their association with bone diseases remain elusive. We have identified and characterized human cOCPs and found a positive association between cOCPs and osteoclast activity. Sorted cOCPs have a higher osteoclastogenic potential than other myeloid cells and effectively differentiate into osteoclasts. cOCPs exhibit distinct morphology and transcriptomic signatures. The frequency of cOCPs in the blood varies among treatment-naive postmenopausal women and has an inverse correlation with lumbar spine bone density and a positive correlation with serum CTX, a bone resorption marker. The increased cOCPs in treatment-naive patients with osteoporosis were significantly diminished by denosumab, a widely used antiresorptive therapy. Our study reveals the distinctive identity of human cOCPs and the potential link between the dynamic regulation of cOCPs and osteoporosis and its treatment. Taken together, our study enhances our understanding of human cOCPs and highlights a potential opportunity to measure cOCPs through a simple blood test, which could potentially identify high-risk individuals.
Keywords: Bone biology; Osteoclast/osteoblast biology; Osteoporosis.
Conflict of interest statement
Figures


References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

