Focal negative motor seizures: Multimodal evaluation
- PMID: 39576184
- PMCID: PMC11742636
- DOI: 10.1111/epi.18191
Focal negative motor seizures: Multimodal evaluation
Abstract
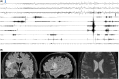
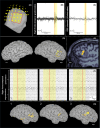
This case report shows the importance of multimodal evaluation to formulate a proper diagnosis of negative motor seizures (NMSs). Only few reports in literature document NMSs with video-electroencephalographic (EEG) and electromyographic coregistration. A multimodal evaluation is crucial to exclude common mimics and propose correct therapy. We describe a case of a 62-year-old man with drug-resistant focal epilepsy and NMSs, evaluated with video-EEG recording with polygraphy, magnetoencephalography (MEG), and brain magnetic resonance imaging (MRI). Video-EEG monitoring showed 182 focal NMSs, with preserved awareness and comprehension. The patient reported complex paresthesia of the left hand followed by left facial grimace, left arm flaccid paralysis, and bradycardia. EEG showed ictal discharges in the right frontocentral region associated with sudden electromyographical silence in left limb muscles consistent with loss of tonic contraction from distal to proximal muscles of the arm. MEG localized the epileptic zone in the right opercular region, consistent with MRI evidence of type II cortical dysplasia in the right inferior frontal gyrus. Multimodal evaluation is essential to document the temporal relationship between ictal discharges, clinical onset of limb paresis, and electrophysiologic evidence of loss of tonic muscular contraction. It allows definition of the specific cortical area involved in NMSs, offering new insight into physiological brain functioning.
Keywords: focal akinetic seizure; ictal bradycardia; ictal paralysis; negative motor area; negative motor seizures; video‐EEG polygraphy.
© 2024 The Author(s). Epilepsia published by Wiley Periodicals LLC on behalf of International League Against Epilepsy.
Conflict of interest statement
The authors declare no conflicts of interest. We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Figures
References
-
- Smyth P. Cortical mapping by electrical stimulation of subdural electrodes: negative motor areas. Textbook of Epilepsy Surgery. London: CRC Press; 2008. p. 1023–1030.
-
- Lüders H, Lesser RP, Dinner DS, Morris HH, Hahn JF, Friedman L, et al. Commentary: chronic intracranial recording and stimulation with subdural electrodes. In: Engel J Jr, editor. Surgical treatment of the epilepsies. New York: Raven Press; 1987. p. 297–321.
-
- Lüders HO, Dinner DS, Morris HH, Wyllie E, Comair YG. Cortical electrical stimulation in humans. The negative motor areas. Adv Neurol. 1995;67:115–129. - PubMed
-
- Fisher M. Left hemiplegia and motor impersistence. J Nerv Ment Dis. 1956;123(3):201–218. - PubMed
-
- Abou‐Khalil B, Fakhoury T, Jennings M, Moots P, Warner J, Kessler RM. Inhibitory motor seizures: correlation with centroparietal structural and functional abnormalities. Acta Neurol Scand. 1995;91(2):103–108. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

