Efanesoctocog Alfa versus Standard and Extended Half-Life Factor VIII Prophylaxis in Adolescent and Adult Patients with Haemophilia A without Inhibitors
- PMID: 39576433
- PMCID: PMC11782424
- DOI: 10.1007/s12325-024-03032-3
Efanesoctocog Alfa versus Standard and Extended Half-Life Factor VIII Prophylaxis in Adolescent and Adult Patients with Haemophilia A without Inhibitors
Abstract
Introduction: In the Phase 3 XTEND-1 trial, (NCT04161495) efanesoctocog alfa prophylaxis provided superior bleed protection versus pre-study factor VIII (FVIII) replacement therapy in patients with severe haemophilia A. The aim of this study was to indirectly compare bleed outcomes between efanesoctocog alfa and standard/extended half-life (SHL and EHL) FVIII replacement therapies in adolescent and adult patients with severe haemophilia A without inhibitors.
Methods: A systematic literature review was conducted to identify Phase 3 trials of EHL and SHL FVIII replacement therapies for comparison with efanesoctocog alfa data from XTEND-1. Matching-adjusted indirect comparisons were used to compare annualised bleeding rates (ABRs) for any, treated, joint, and spontaneous bleeds between efanesoctocog alfa and comparators. The estimates from respective comparisons were pooled using random-effect meta-analyses to evaluate the overall difference between efanesoctocog alfa and comparator therapies.
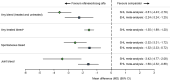
Results: Four EHL therapies (rurioctocog alfa pegol, efmoroctocog alfa, turoctocog alfa pegol, damoctocog alfa pegol) and two octocog alfa SHL therapies were included. In meta-analyses, efanesoctocog alfa was associated with significantly lower ABRs for any [mean difference (95% CI) - 2.24 ( - 3.24; - 1.25)], spontaneous [ - 1.52 ( - 2.33; - 0.72)], and joint bleeds [ - 1.60 ( - 2.32; - 0.88)] versus EHL therapies, and with significantly lower ABRs for any [ - 3.61 ( - 4.43; - 2.79)], treated [ - 1.55 ( - 1.89; - 1.20)], spontaneous [ - 2.52 ( - 3.31; - 1.72)], and joint bleeds [ - 3.42 ( - 4.77; - 2.08)] versus SHL therapies.
Conclusion: Efanesoctocog alfa was associated with significantly lower ABRs (any, spontaneous and joint) compared with EHL or SHL prophylaxis therapies. Patients had, on average, 2.2 and 3.6 fewer bleeds per year versus EHL and SHL therapies, respectively.
Keywords: Annualised bleeding rate; Efanesoctocog alfa; Factor VIII replacement therapies; Haemophilia A; Indirect treatment comparison.
Plain language summary
Factor VIII (FVIII) replacement therapies to prevent bleeding in people with haemophilia A are either standard half-life (SHL) or extended half-life (EHL), and injections may be given two to four times per week. Efanesoctocog alfa is a new FVIII replacement therapy, which only requires a once-weekly injection. In the XTEND-1 clinical trial, people receiving efanesoctocog alfa had fewer bleeds than they did before the trial when they were receiving a different FVIII replacement therapy. However, efanesoctocog alfa has not been compared with available SHL and EHL therapies. Researchers searched medical journals to identify clinical trials of SHL and EHL. They compared the number of bleeds with efanesoctocog alfa in the XTEND-1 trial with each SHL or EHL trial found in the literature search. The results were combined and analysed to find the overall difference between the results with efanesoctocog alfa and each other type of therapy. Compared with SHL and EHL therapy, once-weekly efanesoctocog alfa therapy reduced the rates of any bleeds, including spontaneous (that happen for no apparent reason) and joint bleeds. Overall, people who received efanesoctocog alfa had, on average, 3.6 fewer bleeds per year than those on SHL therapy and 2.2 fewer bleeds per year than those on EHL therapy. As well as reducing the number of injections needed per week, efanesoctocog alfa may work better at preventing bleeds than current SHL or EHL FVIII replacement therapies. This could have a large impact on the lives of people with severe haemophilia A.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Conflict of Interest: Alix Arnaud, Amanda Wilson, and Patricia Guyot are Sanofi employees and may hold stock/stock options in Sanofi. Nana Kragh and Linda Bystrická are employees of Sobi and may hold stock/stock options in Sobi. Piotr Wojciechowski is co-founder at Assignity and an external consultant at Putnam PHMR. Marlena Wdowiak and Wojciech Margas are employees of Putnam PHMR. Robert Klamroth has received honoraria and/or been a member of advisory committees for Bayer, Biomarin, Biotest, CSL Behring, Grifols, Novo Nordisk, Octapharma, Pfizer, Roche/Chugai, Sanofi, Sobi, and Takeda. Alberto Tosetto has no relevant conflicts of interest. Piotr Wojciechowski’s and Wojciech Margas’ affiliation Assignity is now called Clever-Access. Ethical Approval: This is a post hoc analysis and modelling of data already collected and/or published data. Original studies were all approved by the relevant institutional review boards at each study site and were carried out in accordance with the International Conference on Harmonisation good clinical practice guidelines and the Declaration of Helsinki.
Figures

References
-
- Srivastava A, Santagostino E, Dougall A, et al. WFH Guidelines for the Management of Hemophilia, 3rd edition. Haemophilia. 2020;26 Suppl 6:1–158. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

