Metabolic profiling of the synthetic cannabinoid APP-CHMINACA (PX-3) as studied by in vitro and in vivo models
- PMID: 39576558
- PMCID: PMC11782320
- DOI: 10.1007/s11419-024-00705-0
Metabolic profiling of the synthetic cannabinoid APP-CHMINACA (PX-3) as studied by in vitro and in vivo models
Abstract
Purpose: The metabolic pathways of APP-CHMINACA were characterized to select the markers of intake for implementation into analytical assays used by the clinical and forensic communities. We have combined the evidences obtained by both in vitro experiments and administration studies on mice.
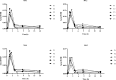
Methods: APP-CHMINACA was incubated with either human or mouse liver microsomes. Urine and blood samples were collected at different time points from mice after injection of a 3 mg/kg dose of the test compound. Samples were analyzed using liquid chromatography-tandem mass spectrometry.
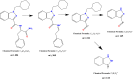
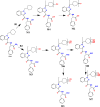
Results: The in vitro studies allowed to isolate eight different metabolic reactions, formed by two metabolic routes, with no differences between human and mouse liver microsomes. The main biotransformation route involved the hydrolysis of the distal amide group and the subsequent hydroxylation on the cyclohexyl-methyl ring. The second route involved multiple hydroxylation of the parent compound, followed by reduction to generate minor metabolites. In blood samples, the most abundant substances identified were APP-CHMINACA unchanged and the metabolites formed by the hydrolysis of the distal amide together with its hydroxylated products. In urine samples, four metabolites formed following the hydroxylation of the distal amide hydrolysis metabolite were detected as the most abundant and long-term metabolites.
Conclusions: The outcomes of our study showed that the most suitable markers to detect the intake of APP-CHMINACA in blood and urine samples in the framework of toxicological, clinical and forensic investigations were the metabolite formed by the hydrolysis of the distal amide and its hydroxylated products.
Keywords: APP-CHMINACA; ICR-CD1 mice; Metabolite identification; PX-3; Synthetic cannabinoids.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Conflict of interest: The authors have no relevant financial or non-financial interests to disclose. Ethical approval: All experimental protocols were in accordance with the U.K. Animals (Scientific Procedures) Act of 1986 and associated guidelines and the new European Communities Council Directive of September 2010 (2010/63/EU), and approved by the Italian Ministry of Health (license n. 223/2021-PR, CBCC2.46.EXT.21) and the Animal Welfare Body of the University of Ferrara. According to the ARRIVE guidelines, all possible efforts were made to minimise the animals’ pain and discomfort and to reduce the number of experimental subjects.
Figures


References
-
- Pertwee RG (2004) Pharmacological and therapeutic targets for Δ9 tetrahydrocannabinol and cannabidiol. Euphytica 140:73–82. 10.1007/s10681-004-4756-9 - DOI
-
- Chimalakonda KC, Seely KA, Bratton SM, Brents LK, Moran CL, Endres GW, James LP, Hollenberg PF, Prather PL, Radominska-Pandya A, Moran JH (2012) Cytochrome P450-mediated oxidative metabolism of abused synthetic cannabinoids found in K2/Spice: identification of novel cannabinoid receptor ligands. Drug Metab Dispos 40:2174–2184. 10.1124/dmd.112.047530 - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

