Apocynaceae wood evolution matches key morphological innovations
- PMID: 39576634
- PMCID: PMC11584039
- DOI: 10.1002/ajb2.16436
Apocynaceae wood evolution matches key morphological innovations
Abstract
Premise: This paper provides an overview of the wood anatomy of the dogbane family (Apocynaceae), reconstructs wood anatomical trait evolution, and links this evolution with woody growth-form transitions and floral and seed trait innovations across the family.
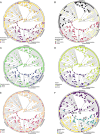
Methods: Over 200 published wood anatomical descriptions were revised, and original light microscopic sections were made and described for another 50 species. Changes in wood anatomical characters through time were visualized with ancestral state reconstructions. Tests for correlated evolution were performed using a combined data set of anatomical and key floral and seed traits to identify potential synnovations and traits associated with growth-form adaptations.
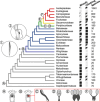
Results: There was a shift toward a suite of wood anatomical traits that separate the rauvolfioids and early-branching apocynoids from the core apocynoids, including an increased presence of vessel multiples, vessel dimorphism, laticifers, vascular (cambial) variants, and paratracheal axial parenchyma. The presence of this trait suite, which continues in Periplocoideae, Secamonoideae, and Asclepiadoideae, coincides with a progression of floral morphological innovations that evolved on consecutive nodes in the family, and also relates to more frequent transitions toward the climbing and herbaceous habits. In addition, a considerable shortening of vessel elements and fibers along the phylogenetic backbone of the family is correlated with a general reduction in plant size.
Conclusions: There are clear evolutionary transitions in the wood anatomy of Apocynaceae representing structural adaptations across the family that are associated with a quick succession of evolutionary changes of the floral bauplan.
Keywords: evolution; lianas; morphology; phylogenetic comparative methods; synnovations; wood anatomy.
© 2024 The Author(s). American Journal of Botany published by Wiley Periodicals LLC on behalf of Botanical Society of America.
Figures


References
-
- Angyalossy, V. , Pace M. R., and Lima A. C.. 2015. Liana anatomy: a broad perspective on structural evolution of the vascular system. In Schnitzer S. A., Bongers F., Burnham R. J., and Putz F. E. [eds.], Ecology of lianas, 251–287. John Wiley & Sons, Chichester, UK.
-
- Antonelli, A. , Clarkson J. J., Kainulainen K., Maurin O., Brewer G. E., Davis A. P., Epitawalage N., et al. 2021. Settling a family feud: a high‐level phylogenomic framework for the Gentianales based on 353 nuclear genes and partial plastomes. American Journal of Botany 108: 1143–1165. - PubMed
-
- Baas, P. , van Heuven B.‐J., Ng X. Y., and Vander Velde N.. 2019. Biomechanical and hydraulic challenges for a tropical swamp forest and driftwood tree – Alstonia spatulata Blume (Apocynaceae). Gardens’ Bulletin Singapore 71: 231–244.
-
- Baas, P. , Werker E., and Fahn A.. 1983. Some ecological trends in vessel characters. IAWA Journal 4: 141–159.
-
- Beckers, V. , Rapini A., Smets E., and Lens F.. 2022. Comparative wood anatomy and origin of woodiness in subfamilies Secamonoideae and Asclepiadoideae (Apocynaceae). Taxon 71: 1230–1250.
MeSH terms
LinkOut - more resources
Full Text Sources

