Glycemic load impacts the response of acquired resistance in breast cancer cells to chemotherapeutic drugs in vitro
- PMID: 39576770
- PMCID: PMC11584130
- DOI: 10.1371/journal.pone.0311345
Glycemic load impacts the response of acquired resistance in breast cancer cells to chemotherapeutic drugs in vitro
Abstract
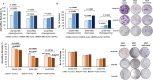
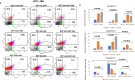
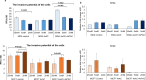
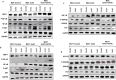
Resisting chemotherapy is a significant hurdle in treating breast cancer. Locally advanced breast cancer patients undergo four cycles of Adriamycin and Cyclophosphamide, followed by four cycles of Paclitaxel before surgery. Some patients resist this regimen, and their cancer recurred. Our study aimed to understand the underlying mechanisms of acquired resistance during these specific treatment phases. We explored how breast cancer cells, resistant to chemotherapy, respond to different glucose levels, shedding light on the intricate relationship between diabetes, breast cancer subtype, and resistance to preoperative chemotherapy. We examined two groups of cell lines: the standard MDA-MB-231 and MCF7 cells and their resistant counterparts after exposure to four cycles of Adriamycin and cyclophosphamide (4xAC) or four cycles of 4xAC and Paclitaxel (4xAC+4xPAC), aiming to unravel the mechanisms and cellular responses at these critical treatment stages. Notably, under normal and low glucose conditions, the resistant MDA-MB-231 cells showed accelerated growth compared to the control cells, while the resistant MCF7 cells proliferated more slowly than their original counterparts. Resistance to 4xAC resulted in significant cell death in both cell lines, especially under low glucose conditions, in contrast to control or 4xAC+4xPAC-resistant cells. The similarity between the MCF7 4xAC+4xPAC resistant cells and the control might be due to the P-AKT expression pattern in response to glucose levels since the levels were constant in MCF7 4xAC in all glucose concentrations. Molecular analysis revealed specific protein accumulations explaining the heightened proliferation and invasion in resistant MDA-MB-231 cells and their ability to withstand low glucose levels compared to MCF7. In conclusion, increased drug involvement corresponds to increased cell resistance, and changes in glucose levels differentially impact resistant variant cells to different drugs. The findings can be translated clinically to explain patients' differential responses to preoperative chemotherapy cycles considering their breast cancer subtype and diabetic status.
Copyright: © 2024 Adham et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

References
-
- Wahdan-Alaswad RS, Thor AD. Metformin activity against breast cancer: mechanistic differences by molecular subtype and metabolic conditions. Metformin: IntechOpen London, UK; 2020.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

