Enhanced dual-mode imaging: Superior photoacoustic and ultrasound endoscopy in live pigs using a transparent ultrasound transducer
- PMID: 39576852
- PMCID: PMC11584001
- DOI: 10.1126/sciadv.adq9960
Enhanced dual-mode imaging: Superior photoacoustic and ultrasound endoscopy in live pigs using a transparent ultrasound transducer
Abstract
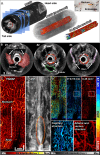
Dual-mode photoacoustic/ultrasound endoscopy (ePAUS) is a promising tool for preclinical and clinical interventions. To be clinically useful, ePAUS must deliver high-performance ultrasound imaging comparable to commercial systems and maintain high photoacoustic imaging performance at long working distances. This requires a transducer with an intact physical aperture and coaxial alignment of acoustic and optical beams within the probe, a challenging integration task. We present a high-performance ePAUS probe with a miniaturized, optically transparent ultrasonic transducer (TUT) called ePAUS-TUT. The 1.8-mm-diameter probe, fitting into standard endoscopic channels, aligns acoustic and optical beams efficiently, achieving commercial-level ultrasound and high-resolution photoacoustic imaging over long distances. These imaging capabilities were validated through in vivo imaging of a rat's rectum and a pig's esophagus. The ePAUS-TUT system substantially enhances feasibility and potential for clinical applications.
Figures



References
-
- Weber J., Beard P. C., Bohndiek S. E., Contrast agents for molecular photoacoustic imaging. Nat. Methods 13, 639–650 (2016). - PubMed
-
- Baik J. W., Kim H., Son M., Choi J., Kim K. G., Baek J. H., Park Y. H., An J., Choi H. Y., Ryu S. Y., Intraoperative label-free photoacoustic histopathology of clinical specimens. Laser Photon. Rev. 15, 2100124 (2021).
-
- Kim J., Park B., Ha J., Steinberg I., Hooper S. M., Jeong C., Park E.-Y., Choi W., Liang T., Bae J. S., Multiparametric photoacoustic analysis of human thyroid cancers in vivo. Cancer Res. 81, 4849–4860 (2021). - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources

