Monoamine transporter ubiquitination and inward-open conformation synergistically maximize transporter endocytosis
- PMID: 39576869
- PMCID: PMC11584022
- DOI: 10.1126/sciadv.adq9793
Monoamine transporter ubiquitination and inward-open conformation synergistically maximize transporter endocytosis
Abstract
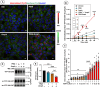
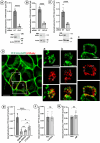
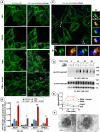
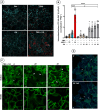
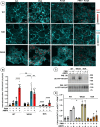
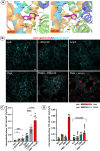
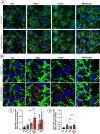
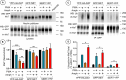
Monoamine transporters function in neuronal membranes to control extracellular concentrations of their substrates. Cell-surface expression of transporters is regulated by substrates and intracellular signaling, but the underlying mechanisms remain unclear. Here, we found that substrates of the dopamine transporter (DAT), amphetamine and dopamine, synergize with protein kinase C (PKC)-dependent DAT ubiquitination to markedly elevate clathrin-mediated endocytosis of DAT, which is accompanied by DAT movement out of plasma membrane protrusions with a negative curvature. Disruption of the outward-open (OO) DAT conformation or its stabilization in the inward-open (IO) conformation recapitulates substrate effects on DAT endocytosis. Amphetamine strongly increases PKC-dependent endocytosis of norepinephrine transporter (NET) but not of serotonin transporter (SERT), correlating with a substantially weaker ubiquitination of SERT compared to NET. We propose a "shape-transition" model whereby shifting from convex-shaped OO conformers to IO conformers minimizes retention of transporters in negatively curved membranes, which facilitates their PKC-dependent ubiquitination and recruitment to positively invaginated clathrin-coated membranes, driving robust transporter endocytosis.
Figures

References
-
- Kristensen A. S., Andersen J., Jorgensen T. N., Sorensen L., Eriksen J., Loland C. J., Stromgaard K., Gether U., SLC6 neurotransmitter transporters: Structure, function, and regulation. Pharm. Rev. 63, 585–640 (2011). - PubMed
-
- Ng J., Papandreou A., Heales S. J., Kurian M. A., Monoamine neurotransmitter disorders—clinical advances and future perspectives. Nat. Rev. Neurol. 11, 567–584 (2015). - PubMed
-
- Pertovaara A., Noradrenergic pain modulation. Prog. Neurobiol. 80, 53–83 (2006). - PubMed
-
- Pignatelli M., Bonci A., Role of dopamine neurons in reward and aversion: A synaptic plasticity perspective. Neuron 86, 1145–1157 (2015). - PubMed
-
- Yildiz O., Smith J. R., Purdy R. E., Serotonin and vasoconstrictor synergism. Life Sci. 62, 1723–1732 (1998). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

