Intermittent or Continuous Panitumumab Plus Fluorouracil, Leucovorin, and Irinotecan for First-Line Treatment of RAS and BRAF Wild-Type Metastatic Colorectal Cancer: The IMPROVE Trial
- PMID: 39576946
- PMCID: PMC11856000
- DOI: 10.1200/JCO.24.00979
Intermittent or Continuous Panitumumab Plus Fluorouracil, Leucovorin, and Irinotecan for First-Line Treatment of RAS and BRAF Wild-Type Metastatic Colorectal Cancer: The IMPROVE Trial
Abstract
Purpose: To investigate whether intermittent treatment after an induction phase of first-line schedule of fluorouracil, leucovorin, and irinotecan (FOLFIRI) plus panitumumab (PAN) prevents or delays the onset of resistance and improves safety and compliance with treatment in patients with unresectable RAS/BRAF wild-type (wt) metastatic colorectal cancer (mCRC).
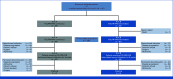
Patients and methods: IMPROVE (ClinicalTrials.gov identifier: NCT04425239) was an open-label, multicenter, randomized phase II noncomparative trial. Patients with unresectable RAS/BRAF wt mCRC were randomly assigned (1:1) to receive FOLFIRI plus PAN continuously until progression (arm A) or intermittently, with treatment-free intervals (arm B) until progression on treatment, toxicity, or death. The primary end point was progression-free survival on treatment (PFSot) at 12 months. Assuming a null hypothesis of median PFSot time ≤7 months and target PFSot ≥10 months, 65 patients per arm were needed to achieve 80% power and 10% type I error, according to the binomial test.
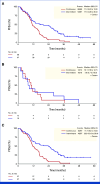
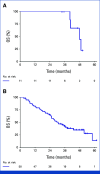
Results: Between May 2018 and June 2021, 69 patients were randomly assigned to arm A and 68 to arm B. The median number of treatment cycles was 13 in arm A and 16 in arm B. At a median follow-up of 43.2 months (IQR, 35.0-50.5), median PFSot was 11.2 and 17.5 months with 12-month PFSot rates of 45.7% and 58.5%, for arms A and B, respectively. The overall response rates were 68.1% and 61.2%, and median overall survival rates were 36.3 and 35.1 months in arms A and B, respectively. The overall rate of grade >2 skin PAN-related adverse events was 30.3% in arm A and 17.9% in arm B.
Conclusion: Intermittent FOLFIRI plus PAN after the induction phase was feasible, and the primary end point was met with reduced toxicity while allowing patients more time off treatment.
Conflict of interest statement
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (
No other potential conflicts of interest were reported.
Figures


References
-
- Morgan E, Arnold M, Gini A, et al. : Global burden of colorectal cancer in 2020 and 2040: Incidence and mortality estimates from GLOBOCAN. Gut 72:338-344, 2023 - PubMed
-
- Biller LH, Schrag D: Diagnosis and treatment of metastatic colorectal cancer: A review. JAMA 325:669-685, 2021 - PubMed
-
- Cervantes A, Adam R, Rosello S, et al. : Metastatic colorectal cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann Oncol 34:10-32, 2023 - PubMed
-
- Di Nicolantonio F, Vitiello PP, Marsoni S, et al. : Precision oncology in metastatic colorectal cancer—From biology to medicine. Nat Rev Clin Oncol 18:506-525, 2021 - PubMed
-
- Siravegna G, Mussolin B, Buscarino M, et al. : Clonal evolution and resistance to EGFR blockade in the blood of colorectal cancer patients. Nat Med 21:827, 2015 - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

