MRD-driven phase 2 study of daratumumab, carfilzomib, lenalidomide, and dexamethasone in newly diagnosed multiple myeloma
- PMID: 39576965
- PMCID: PMC11814523
- DOI: 10.1182/bloodadvances.2024014417
MRD-driven phase 2 study of daratumumab, carfilzomib, lenalidomide, and dexamethasone in newly diagnosed multiple myeloma
Abstract
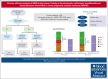
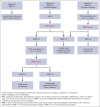
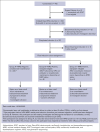
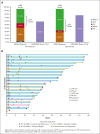
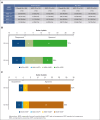
In newly diagnosed multiple myeloma (NDMM), measurable residual disease (MRD) status is prognostically important, but its role in treatment decisions remains unclear. In a phase 2 trial, we assessed daratumumab, carfilzomib, lenalidomide, and dexamethasone (Dara-KRd) induction followed by a next-generation sequencing-based MRD-adapted strategy. The primary outcome was complete response (CR) and stringent CR (≥CR) after induction. Flow cytometry was used to profile T cells. Among 39 patients, 21 (54%) achieved ≥CR after induction (P = .375), with MRD-negative rates of 59% (10-5) and 41% (10-6). Patients who were MRD-negative (n = 24, group A) received lenalidomide maintenance, showing sustained MRD negativity in 14 of 18 (77.8%) for ≥12 cycles. MRD-positive transplant-eligible patients (n = 8, group B) underwent autologous stem cell transplantation, with 62.5% converting to MRD-negative at 10-5 (37.5% at 10-6) posttransplant. MRD-positive, transplant-ineligible patients (n = 4, group C) received KRd consolidation. Best MRD-negative rates improved to 77% (10-5) and 72% (10-6). No new safety concerns were identified for Dara-KRd. With a median follow-up of 30.1 months, 3, 2, and 1 patient(s) in groups A, B, and C, respectively, have progressed or died. We observed that Dara-KRd strongly activated memory T cells, which was associated with an MRD-negative state post induction. Although the primary outcome was not met, Dara-KRd induction in NDMM achieved high ≥CR and MRD-negative rates without new safety concerns. The post induction MRD-adapted strategy deepened responses in MRD-positive patients and maintained durable MRD control in MRD-negative patients. This trial was registered at www.clinicaltrials.gov as #NCT04113018.
© 2025 American Society of Hematology. Published by Elsevier Inc. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: M.B. reports research funding (to institute) from Janssen, Amgen, Bristol Myers Squibb/Celgene, Takeda, and Caribou Biosciences. S.A. reports honoraria and research funding from GlaxoSmithKline, Amgen, and Karyopharm; and honoraria from Janssen. B.P. reports membership on an entity's board of directors or advisory committees with AbbVie and Janssen; and research funding (to institute) from Bristol Myers Squibb. J.T.S. reports consultancy with Astellas Pharma, Camurus, Eli Lilly and Co, CARsgen, Fusion Pharmaceuticals, Jazz Pharmaceuticals, and Immatics. P.M.V. reports consultancy with, and honoraria from, AbbVie, Amgen, Bristol Myers Squibb, GlaxoSmithKline, Karyopharm, Novartis, Oncopeptides, Pfizer, Sanofi, and SecuraBio. S.Z.U. reports research funding from AbbVie, Amgen, Bristol Myers Squibb, Celgene, EdoPharma, Genentech, Gilead, GlaxoSmithKline, Janssen Pharmaceuticals, Oncopeptides, Sanofi, SeaGen Inc (formerly Seattle Genetics, Inc), Secura Bio Inc, SkylineDx, Takeda, TeneoBio, Amgen, Array Biopharma, Bristol Myers Squibb, Celgene, GlaxoSmithKline, and Janssen; consultancy with Amgen, Array Biopharma, Bristol Myers Squibb, Celgene, GlaxoSmithKline, Janssen Pharmaceuticals, Merck, Pharmacyclics, Sanofi, SeaGen Inc (formerly Seattle Genetics, Inc), SkylineDx, and Takeda; and serving on the speakers bureau of Amgen, Bristol Myers Squibb, Janssen Pharmaceuticals, and Sanofi. The remaining authors declare no competing financial interests.
Figures

References
-
- Costa LJ, Chhabra S, Medvedova E, et al. Daratumumab, carfilzomib, lenalidomide, and dexamethasone with minimal residual disease response-adapted therapy in newly diagnosed multiple myeloma. J Clin Oncol. 2022;40(25):2901–2912. - PubMed
-
- Voorhees PM, Sborov DW, Laubach J, et al. Addition of daratumumab to lenalidomide, bortezomib, and dexamethasone for transplantation-eligible patients with newly diagnosed multiple myeloma (GRIFFIN): final analysis of an open-label, randomised, phase 2 trial. Lancet Haematol. 2023;10(10):e825–e837. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

