Urine-Xpert Ultra for the diagnosis of tuberculosis in people living with HIV: a prospective, multicentre, diagnostic accuracy study
- PMID: 39577975
- PMCID: PMC11584317
- DOI: 10.1016/S2214-109X(24)00357-7
Urine-Xpert Ultra for the diagnosis of tuberculosis in people living with HIV: a prospective, multicentre, diagnostic accuracy study
Abstract
Background: Diagnostic delays for tuberculosis are common, with high resultant mortality. Urine-Xpert Ultra (Cepheid) could improve time to diagnosis of tuberculosis disease and rifampicin resistance. We previously reported on lot-to-lot variation of the Fujifilm SILVAMP TB LAM. In this prespecified secondary analysis of the same cohort, we aimed to determine the diagnostic yield and accuracy of Urine-Xpert Ultra for tuberculosis in people with HIV, compared with an extended microbiological reference standard (eMRS) and composite reference standard (CRS) and also compared with Determine TB LAM Ag (AlereLAM, Abbott).
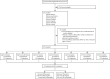
Methods: In this prospective, multicentre, diagnostic accuracy study, we recruited consecutive inpatients and outpatients (aged ≥18 years) with HIV from 13 hospitals and clinics in seven countries (Malawi, South Africa, Tanzania, Thailand, Uganda, Viet Nam, and Zambia). Patients with no isoniazid preventive therapy in the past 6 months and fewer than three doses of tuberculosis treatment in the past 60 days were included. Reference and index testing was performed in real time. The primary outcome of this secondary analysis was the diagnostic yield and accuracy of Urine-Xpert Ultra compared with the eMRS and CRS. Diagnostic accuracy was compared with AlereLAM and diagnostic yield was compared with both AlereLAM and Sputum-Xpert Ultra. This study was registered with ClinicalTrials.gov, NCT04089423, and is complete.
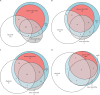
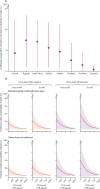
Findings: Between Dec 13, 2019, and Aug 5, 2021, 3528 potentially eligible individuals were screened and 1731 were enrolled, of whom 1602 (92·5%) were classifiable by the eMRS (median age 40 years [IQR 33-48], 838 [52·3%] of 1602 were female, 764 [47·7%] were male, 937 [58·5%] were outpatients, 665 [41·5%] were inpatients, median CD4 count was 374 cells per μL [IQR 138-630], and 254 [15·9%] had microbiologically confirmed tuberculosis). Against eMRS as reference, sensitivities of Urine-Xpert Ultra and AlereLAM were 32·7% (95% CI 27·2-38·7) and 30·7% (25·4-36·6) and specificities were 98·0% (97·1-98·6) and 90·4% (88·7-91·8), respectively. Against CRS as reference, sensitivities of Urine-Xpert Ultra and AlereLAM were 21·1% (95% CI 17·6-25·1), and 30·5% (26·4-34·9), and specificities were 99·1% (98·3-99·6) and 95·1% (93·5-96·3), respectively. The combination of Sputum-Xpert Ultra with AlereLAM or Urine-Xpert Ultra diagnosed 202 (77·1%) and 204 (77·9%) of 262 eMRS-positive participants, respectively, in incompletely overlapping groups; combining all three tests diagnosed 214 (81·7%) of 262 eMRS-positive participants INTERPRETATION: Urine-Xpert Ultra could offer promising clinical utility in addition to AlereLAM and Sputum-Xpert Ultra. In inpatient settings where both AlereLAM and Urine-Xpert Ultra are possible, both should be offered to support rapid diagnosis and treatment.
Funding: Global Health Innovative Technology Fund, KfW Development Bank, Commonwealth of Australia represented by the Department of Foreign Affairs and Trade, and the Netherlands Enterprise Agency.
Copyright © 2024 The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY 4.0 license. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Declaration of interests RS, BE, CMD, and MR are or were employed by FIND, the global alliance for diagnostics, at the time of the study; FIND is a not-for-profit foundation that supports the evaluation of publicly prioritised tuberculosis assays and the implementation of WHO-approved (guidance and prequalification) assays using donor grants; FIND has product evaluation agreements with several private sector companies that design diagnostics for tuberculosis and other diseases; these agreements strictly define FIND's independence and neutrality with regard to these private sector companies. CMD reports funding from the US National Institutes of Health for the R2D2 project (1U01AI152087-01). All other authors declare no competing interests.
Figures


References
-
- WHO . World Health Organization; Geneva: 2022. Global tuberculosis report 2022.https://apps.who.int/iris/handle/10665/363752
-
- Ford N, Shubber Z, Meintjes G, et al. Causes of hospital admission among people living with HIV worldwide: a systematic review and meta-analysis. Lancet HIV. 2015;2:e438–e444. - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

