Vascular dysfunction is at the onset of oxaliplatin-induced peripheral neuropathy symptoms in mice
- PMID: 39578077
- PMCID: PMC11584327
- DOI: 10.26508/lsa.202402791
Vascular dysfunction is at the onset of oxaliplatin-induced peripheral neuropathy symptoms in mice
Abstract
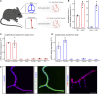
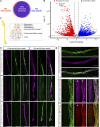
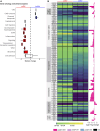
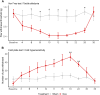
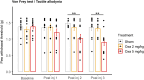
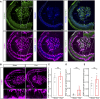
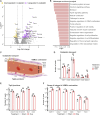
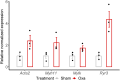
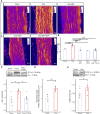
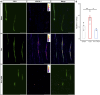
Oxaliplatin-induced peripheral neuropathy (OIPN) is an adverse side effect of this chemotherapy used for gastrointestinal cancers. The continuous pain experienced by OIPN patients often results in the reduction or discontinuation of chemotherapy, thereby affecting patient survival. Several pathogenic mechanisms involving sensory neurons were shown to participate in the occurrence of OIPN symptoms. However, the dysfunction of the blood-nerve barrier as a source of nerve alteration had not been thoroughly explored. To characterise the vascular contribution to OIPN symptoms, we undertook two comparative transcriptomic analyses of mouse purified brain and sciatic nerve blood vessels (BVs) and nerve BVs after oxaliplatin or control administration. These analyses reveal distinct molecular landscapes between brain and nerve BVs and the up-regulation of transcripts involved in vascular contraction after oxaliplatin treatment. Anatomical examination of the nerve yet shows the preservation of BV architecture in the acute OIPN mouse model, although treated mice exhibit both neuropathic symptoms and enhanced vasoconstriction reflected by hypoxia. Moreover, vasodilators significantly reduce oxaliplatin-induced neuropathic symptoms and endoneurial hypoxia, establishing the key involvement of nerve blood flow in OIPN.
© 2024 Taïb et al.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures



References
-
- Argyriou AA, Cavaletti G, Briani C, Velasco R, Bruna J, Campagnolo M, Alberti P, Bergamo F, Cortinovis D, Cazzaniga M, et al. (2013) Clinical pattern and associations of oxaliplatin acute neurotoxicity: A prospective study in 170 patients with colorectal cancer. Cancer 119: 438–444. 10.1002/cncr.27732 - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
