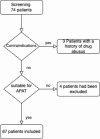
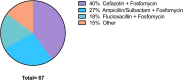
Peripherally inserted central venous catheter in outpatient antibiotic spinal infection treatment is safe, effective and leads to significant reduction in healthcare expenses
- PMID: 39578285
- PMCID: PMC11584477
- DOI: 10.1007/s10143-024-03127-z
Peripherally inserted central venous catheter in outpatient antibiotic spinal infection treatment is safe, effective and leads to significant reduction in healthcare expenses
Abstract
Prolonged antibiotic therapy is often recommended for the treatment of spinal infections. This study aimed to evaluate the efficacy and safety of outpatient intravenous (IV) antibiotic therapy for spinal neurosurgery patients with spondylodiscitis. We carried out a retrospective study involving 67 patients who were administered peripherally inserted central catheter (PICC) for IV antibiotic treatment from January 2020 to December 2022. We assessed patient data concerning infections and neurosurgical concerns. Each patient underwent a minimum of 6 weeks of IV antibiotics, both as inpatients and outpatients. The study included 67 patients with a median age of 61 years (SD +/- 14.18 years), with approximately 44% being female. The average hospital stay for inpatient treatment was 20 days (SD +/- 8.8 days). Subsequent outpatient antibiotic therapy lasted an average of 70.32 days (SD +/- 18.24 days), with outpatient IV therapy accounting for 44.74 days (SD +/- 9.15 days). The most common pathogens identified were Staphylococcus epidermidis and methicillin-sensitive Staphylococcus aureus. Microbiological analysis did not detect any pathogens in 18% of patients. Radiographic and laboratory evidence of spondylodiscitis was absent in 99% of patients during the final follow-up. No catheter-related complications occurred. Outpatient IV antibiotic therapy using a PICC line catheter is a safe and effective treatment option for spinal infections, especially in elderly patients.
Keywords: Complications; Efficacy; Intravenous antibiotic therapy; Outpatient; Peripherally inserted central catheter (PICC); Safety; Spinal infection; Spondylodiscitis.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Ethical approval: The study protocol was approved by the ethics committee of the Ludwig-Maximilian-University Munich (Bavaria, Germany) on May 26th, 2023, reference number 2023 − 0304. Consent to participate: All patients provided informed consent for participation in the study. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures
References
-
- Baryeh K, Anazor F, Iyer S, Rajagopal T (2022) Spondylodiscitis in adults: diagnosis and management. Br J Hosp Med (Lond) 83:1–9 - PubMed
-
- Lawson McLean A, Senft C, Schwarz F (2023) Management of lumbar pyogenic spondylodiscitis in Germany: a cross-sectional analysis of spine specialists. World Neurosurg 173:e663–e668 - PubMed
-
- Bernard L et al (2015) Antibiotic treatment for 6 weeks versus 12 weeks in patients with pyogenic vertebral osteomyelitis: an open-label, non-inferiority, randomised, controlled trial. Lancet 385:875–882 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical