Bimekizumab Efficacy in High-Impact Areas: Pooled 2-Year Analysis in Scalp, Nail, and Palmoplantar Psoriasis from Phase 3/3b Randomized Controlled Trials
- PMID: 39578348
- PMCID: PMC11604908
- DOI: 10.1007/s13555-024-01295-w
Bimekizumab Efficacy in High-Impact Areas: Pooled 2-Year Analysis in Scalp, Nail, and Palmoplantar Psoriasis from Phase 3/3b Randomized Controlled Trials
Abstract
Introduction: Psoriasis in high-impact areas, including the scalp, nails, palms, and soles, can disproportionately impair patient quality of life. Here, we evaluate the 2-year efficacy of bimekizumab treatment in patients with moderate to severe plaque psoriasis in post hoc analyses of five phase 3/3b trials.
Methods: High-impact area efficacy data were pooled through 2 years across five phase 3/3b trials: BE VIVID, BE READY, BE SURE, their ongoing open-label extension (OLE) BE BRIGHT, and BE RADIANT (including its double-blinded treatment period and the first year of its OLE). Complete clearance of psoriasis in high-impact areas is reported over 2 years using the scalp Investigator's Global Assessment (IGA), palmoplantar IGA, and modified Nail Psoriasis Severity Index (mNAPSI). Patients included in these analyses had baseline moderate to severe scalp or palmoplantar involvement (scalp or palmoplantar IGA score ≥ 3) or mNAPSI score > 10.
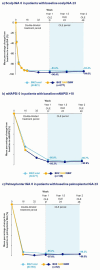
Results: A total of 1107 patients were randomized to bimekizumab and entered the OLEs. Subsets of 821 patients had scalp IGA ≥ 3 at baseline, 377 had mNAPSI > 10, and 193 had palmoplantar IGA ≥ 3. Complete scalp clearance in patients with baseline scalp IGA ≥ 3 randomized to bimekizumab was achieved rapidly, with high responses sustained from first (86.4%) to second year (85.9%). Nail clearance responses in patients with baseline mNAPSI > 10 increased from 63.4% to 68.5% from first to second year. Palmoplantar clearance in patients with baseline palmoplantar IGA ≥ 3 was sustained from first (88.3%) to second year (89.8%). Similar trends were seen in the 374 patients who received bimekizumab 320 mg every 4 weeks (Q4W)/every 8 weeks (Q8W) initial/maintenance dosing.
Conclusion: In these analyses pooled across 2 years, bimekizumab showed sustained efficacy in psoriasis in high-impact areas.
Gov trial registration numbers: NCT03370133, NCT03410992, NCT03412747, NCT03598790, NCT03536884.
Keywords: Bimekizumab; Clinical trial; Efficacy; High-impact areas; Nail; Palmoplantar; Palms; Psoriasis; Scalp; Soles.
Plain language summary
Psoriasis in some body areas can have a bigger impact on the self-confidence and well-being of patients. These body areas, called high-impact areas, are often very visible or important for day-to-day activities. They include the scalp, fingernails, palms, and soles of the feet. People with psoriasis often find applying creams or ointments to these areas challenging. The treatment may also not be effective. Therefore, new medications that can clear psoriasis from these areas are needed by patients and physicians. Bimekizumab is a drug given by injection. We examined whether bimekizumab can clear psoriasis in high-impact areas over 2 years in five clinical trials. Psoriasis of the scalp, palms, and soles cleared quickly with bimekizumab. Most patients reported clear skin in these areas after 4 months, and skin remained clear for the rest of the 2-year period. After 2 years, 90% (18 in 20) of patients with psoriasis on their palms and soles saw it clear completely; 86% of patients (around 17 in 20) saw their scalp psoriasis completely cleared. Nail psoriasis took slightly longer to clear, because nails grow more slowly. Nevertheless, 63% of patients (around 13 in 20) had completely clear nails after 1 year and 69% of patients (around 14 in 20) had clear nails after 2 years. Bimekizumab can clear psoriasis in high-impact areas quickly, and this is maintained over the long-term. Bimekizumab can provide a lasting treatment option for areas of the body which are difficult to treat and have a big impact on patients’ lives.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Conflicts of Interest: All details of authors’ affiliation or involvement in an organization or entity with a financial or nonfinancial interest in the subject matter or materials discussed in this manuscript are disclosed. Joseph F. Merola: A consultant and/or investigator for AbbVie, Amgen, Biogen, Bristol Myers Squibb, Dermavant, Eli Lilly, Janssen, LEO Pharma, Pfizer, Novartis, Regeneron, Sanofi, Sun Pharma, and UCB. Alice B. Gottlieb: Received honoraria as an advisory board member and consultant for Amgen, AnaptypsBio, Avotres Therapeutics, Bristol Myers Squibb, Boehringer Ingelheim, Dice Therapeutics, Eli Lilly, Janssen, Novartis, Sanofi, UCB, and Xbiotech; received research/educational grants from AnaptypsBio, Bristol Myers Squibb, Moonlake Immunotherapeutics, Novartis, and UCB; all funds paid to Mount Sinai School of Medicine. Andreas Pinter: Investigator and/or speaker and/or advisor for AbbVie, Almirall, Amgen, Biogen, Boehringer Ingelheim, Celgene, Eli Lilly, Galderma, GSK, Hexal, Janssen, LEO Pharma, MC2, Medac, Merck Serono, Mitsubishi Pharma, MSD, MoonLake Immunotherapeutics, Novartis, Pfizer, Regeneron, Roche, Sandoz, Schering-Plough, Tigercat Pharma, and UCB. Boni Elewski: Research support as funding to Case Western Reserve University from AbbVie, AnaptysBio, Boehringer Ingelheim, Bristol Myers Squibb, Celgene, Eli Lilly, Incyte, LEO Pharma, Menlo, Merck, Novartis, Pfizer, Regeneron, Sun Pharma, Valeant, and Vanda; Consultant (honoraria) from Arcutis, Boehringer Ingelheim, Bristol Myers Squibb, Celgene, Eli Lilly, LEO Pharma, Menlo, Novartis, Pfizer, Sun Pharma, UCB, Valeant, and Verrica. Melinda Gooderham: Investigator, speaker, consultant, or advisory board member for AbbVie, Akros, Amgen, AnaptysBio, Arcutis, Aslan, Aristea, Bausch Health, Boehringer Ingelheim, Bristol Myers Squibb, Celgene, Dermavant, Dermira, Eli Lilly, Galderma, GSK, Incyte, Janssen, Kyowa Kirin, MedImmune, Meiji, Merck, Moonlake Immunotherapeutics, Nimbus, Novartis, Pfizer, Regeneron, Reistone, Sanofi Genzyme, Sun Pharma, and UCB. Richard B. Warren: Consulting fees from AbbVie, Almirall, Amgen, Arena, Astellas, Avillion, Biogen, Boehringer Ingelheim, Bristol Myers Squibb, Celgene, Eli Lilly, GSK, Janssen, LEO Pharma, Novartis, Pfizer, Sanofi, and UCB; research grants to institution from AbbVie, Almirall, Janssen, LEO Pharma, Novartis, and UCB; honoraria from Astellas, DICE Therapeutics, GSK, and Union Therapeutics. Stefano Piaserico: Served as consultant and/or speaker for AbbVie, Almirall, Celgene, Eli Lilly, Janssen, LEO Pharma, Merck, Novartis, Pfizer, Sandoz, and UCB. Krista Wixted, Nancy Cross, Nicola Tilt, Susanne Wiegratz: Employees and shareholders of UCB. Ulrich Mrowietz: Advisor and/or clinical study investigator for, and/or received honoraria and/or grants from AbbVie, Aditxt, Almirall, Amgen, Aristea, Biogen, Boehringer Ingelheim, Bristol Myers Squibb, Celgene, Dr. Reddy’s, Eli Lilly, Formycon, Immunic, Janssen-Cilag, LEO Pharma, Merck, MetrioPharm, Novartis, Phi-Stone, Sanofi-Aventis, Merck Sharp & Dohme, UCB, and Union Therapeutics. Ethical Approval: Reviewed and approved by the relevant IRBs. Clinicaltrials.gov trial registration: NCT03370133, NCT03410992, NCT03412747, NCT03598790, NCT03536884. Studies were conducted in accordance with the principles of the Declaration of Helsinki and approved by an independent review board and independent ethics committee. All participants provided informed written consent documented in accordance with local regulations. Written consent for the publication of recognizable patient photographs or other identifiable material was obtained by the authors and attested to at the time of article submission to the journal stating that all patients gave consent with the understanding that this information may be publicly available.
Figures

References
-
- Augustin M, Sommer R, Kirsten N, et al. Topology of psoriasis in routine care: results from high-resolution analysis of 2009 patients. Br J Dermatol. 2019;181(2):358–65. - PubMed
-
- Mrowietz U, Augustin M. Using the upgrade criteria of the European psoriasis consensus is best practice care according to the people-centred healthcare concept of the World Health Organization. Br J Dermatol. 2022;187(6):1007–8. - PubMed
-
- Eghlileb AM, Davies EE, Finlay AY. Psoriasis has a major secondary impact on the lives of family members and partners. Br J Dermatol. 2007;156(6):1245–50. - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

