Phage cocktail amikacin combination as a potential therapy for bacteremia associated with carbapenemase producing colistin resistant Klebsiella pneumoniae
- PMID: 39578508
- PMCID: PMC11584731
- DOI: 10.1038/s41598-024-79924-9
Phage cocktail amikacin combination as a potential therapy for bacteremia associated with carbapenemase producing colistin resistant Klebsiella pneumoniae
Abstract
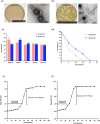
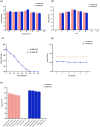
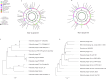
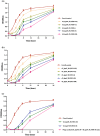
The increasing occurrence of hospital-associated infections, particularly bacteremia, caused by extensively drug-resistant (XDR) carbapenemase-producing colistin-resistant Klebsiella pneumoniae highlights a critical requirement to discover new therapeutic alternatives. Bacteriophages having host-specific bacteriolytic effects are promising alternatives for combating these pathogens. Among 12 phages isolated from public wastewater in Thailand, two phages-vB_kpnM_05 (myovirus) and vB_kpnP_08 (podovirus) showed broad-host range, producing bacteriolytic activities against 81.3% (n = 26) and 78.1% (n = 25) of 32 XDR carbapenemase-producing colistin-resistant K. pneumoniae, with capsular types-K15, K17, K50, K51, K52/wzi-50 and K2/wzi-2. Both phages showed short replication times, large burst sizes with rapid adsorptions. They exhibited significant stability under various environmental conditions. Genomic analysis revealed that both phages are genetically distinct phages from Myoviridae and Podoviridae family, with the lack of toxin, virulence, lysogeny and antibiotic resistance genes. These characteristics highlighted their promising potential for utilizing in phage therapy for combating XDR K. pneumoniae. Although phage cocktail combining vB_kpnM_05 and vB_kpnP_08 provided significant bacteriolysis for longer duration (8 h) than its monophage (6 h), bacterial regrowth was observed which suggested an evitable development of phage resistance under phages' selection pressures. Future study will be undertaken to elucidate the precise mechanisms by which these XDR K. pneumoniae developed phage resistance and their associated fitness cost. Remarkably, combining phage cocktail with amikacin at their sub-inhibitory concentrations produced potent synergy by completely suppressing bacterial regrowth in vitro. Our study demonstrated the significant therapeutic and prophylactic effectiveness of a phage cocktail-amikacin combination as a promising alternative strategy for overcoming bacteremia associated with XDR K. pneumoniae having carbapenemase and colistin resistance in vivo.
Keywords: Klebsiella pneumoniae; Bacteremia; Carbapenemase; Colistin resistance; Extensively drug-resistant; Phage cocktail; Phage cocktail-antibiotic combination.
© 2024. The Author(s).
Conflict of interest statement
Declaration. Competing interests: The authors declare no competing interests. Informed consent: For this retrospective study of anonymous clinical isolates, the requirement for informed consent from patients was waived by the Institutional Review Board (IRB) of the Faculty of Medicine, Chulalongkorn University, Bangkok, Thailand (IRB No.2865/65).
Figures



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

