The effects of WWP1 overexpression on the golgi apparatus stress response and proteoglycan production in adipocytes
- PMID: 39578509
- PMCID: PMC11584891
- DOI: 10.1038/s41598-024-79114-7
The effects of WWP1 overexpression on the golgi apparatus stress response and proteoglycan production in adipocytes
Abstract
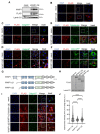
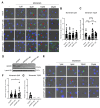
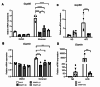
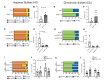
White adipocytes are a major component of white adipose tissue (WAT) and help to maintain systemic metabolic homeostasis by storing energy and secreting adipokines. In mice deficient in the protein WWP1 (WW domain-containing E3 ubiquitin protein ligase 1), oxidative stress in adipocytes increases but insulin resistance induced by obesity improves. However, the specific roles of WWP1 in adipocytes remain unclear. Here, we show that in 3T3L1 adipocytes, WWP1 localized in the Golgi apparatus via its C2 domain, where it protected the Golgi apparatus from monensin-induced disruption. By contrast, WWP1 knockdown by short hairpin RNA failed to protect the Golgi apparatus and enhanced Golgi apparatus disruption by monensin. The Golgi apparatus acts as a central organelle to establish accurate protein glycosylation of proteoglycans containing glycosaminoglycans, including chondroitin sulfate and heparan sulfate (HS). WWP1 overexpression increased chondroitin sulfate and HS levels, whereas WWP1 knockdown decreased them. Furthermore, obesity-related increases in HS were prevented by WWP1 knockout in adipose tissue. In summary, our results demonstrate a novel role for WWP1 in maintaining Golgi apparatus morphology and proteoglycan synthesis in adipocytes.
Keywords: Adipose tissue; Glycosaminoglycan; Golgi apparatus; WWP1.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests.
Figures
Similar articles
-
Identification of WWP1 as an obesity-associated E3 ubiquitin ligase with a protective role against oxidative stress in adipocytes.Biochem Biophys Res Commun. 2019 Jan 1;508(1):117-122. doi: 10.1016/j.bbrc.2018.11.127. Epub 2018 Nov 22. Biochem Biophys Res Commun. 2019. PMID: 30471861
-
WWP1 knockout in mice exacerbates obesity-related phenotypes in white adipose tissue but improves whole-body glucose metabolism.FEBS Open Bio. 2020 Mar;10(3):306-315. doi: 10.1002/2211-5463.12795. Epub 2020 Feb 3. FEBS Open Bio. 2020. PMID: 31965758 Free PMC article.
-
Depletion of WWP1 Increases Adrb3 Expression and Lipolysis in White Adipose Tissue of Obese Mice.Int J Mol Sci. 2025 Apr 29;26(9):4219. doi: 10.3390/ijms26094219. Int J Mol Sci. 2025. PMID: 40362456 Free PMC article.
-
Proteoglycan synthesis and Golgi organization in polarized epithelial cells.J Histochem Cytochem. 2012 Dec;60(12):926-35. doi: 10.1369/0022155412461256. Epub 2012 Sep 1. J Histochem Cytochem. 2012. PMID: 22941419 Free PMC article. Review.
-
WWP1: a versatile ubiquitin E3 ligase in signaling and diseases.Cell Mol Life Sci. 2012 May;69(9):1425-34. doi: 10.1007/s00018-011-0871-7. Epub 2011 Nov 4. Cell Mol Life Sci. 2012. PMID: 22051607 Free PMC article. Review.
Cited by
-
rTMS ameliorates cerebral ischemia-reperfusion injury by inhibiting Golgi apparatus stress through epigenetic modulation of Gli2.Commun Biol. 2025 Aug 13;8(1):1209. doi: 10.1038/s42003-025-08613-8. Commun Biol. 2025. PMID: 40804340 Free PMC article.
References
-
- Hotamisligil, G. S. Inflammation and metabolic disorders. Nature. 444, 860–867. 10.1038/nature05485 (2006). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

