Outcomes, safety and health economics of introduction of video laryngoscopy-assisted less invasive surfactant administration
- PMID: 39578512
- PMCID: PMC12069099
- DOI: 10.1038/s41372-024-02162-4
Outcomes, safety and health economics of introduction of video laryngoscopy-assisted less invasive surfactant administration
Abstract
Background: Less invasive surfactant administration (LISA) is associated with better outcomes than InSurE (Intubation-Surfactant administration-Extubation). Video-laryngoscopy (VL) facilitates intubation in neonates, however safety and cost-effectiveness of VL-assisted LISA have not been evaluated.
Methods: We compared the outcomes of infants receiving VL-assisted LISA (n = 67) with a historical cohort of infants who received InSurE (n = 52). Secondary aims were to evaluate safety and cost-effectiveness.
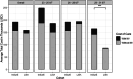
Results: VL-assisted LISA was associated with reduced duration of non-invasive ventilation (NIV), reduced duration of oxygen therapy, reduced composite days on NIV and mechanical ventilation (MV), and shorter NICU stay with lower hospital costs for infants ≥29 weeks GA, compared to InSurE. In the VL-assisted LISA group, 66% of the tracheal catheters were placed on the first attempt and 16% of infants displayed desaturation during placement.
Conclusion: In infants ≥29 weeks GA, VL-assisted LISA reduced exposure to NIV, oxygen, NIV and MV combined, length of stay, and cost of care compared to InSurE.
© 2024. The Author(s).
Conflict of interest statement
Competing interests: Dalibor Kurepa has been clinical consultant for Chiesi Farmaceutici S.p.A. since April 2024. This company produces surfactants, however Chiesi Farmaceutici S.p.A. had no role in the production of this manuscript and is unrelated to the present work. Ethics approval and consent to participate: The study was approved by the Northwell Health IRB (Feinstein Institutes, ID-21-1127-CCMC) as a quality improvement project, therefore no patient informed consent was needed for data collection.
Figures
Similar articles
-
The influence of the technique of surfactant administration (LISA vs INSURE) on the outcomes of respiratory distress syndrome treatment in preterm infants.Dev Period Med. 2019;23(3):163-171. doi: 10.34763/devperiodmed.20192303.163171. Dev Period Med. 2019. PMID: 31654994 Free PMC article.
-
Less Invasive Surfactant Administration (LISA) versus Intubation Surfactant Extubation (InSurE) technique using higher volume surfactant in management of neonates with respiratory distress syndrome: an open-label randomized controlled trial.Eur J Pediatr. 2025 May 29;184(6):371. doi: 10.1007/s00431-025-06191-9. Eur J Pediatr. 2025. PMID: 40439732 Clinical Trial.
-
Surfactant administration methods for premature newborns: LISA vs. INSURE comparative analysis.J Neonatal Perinatal Med. 2024;17(2):233-239. doi: 10.3233/NPM-230194. J Neonatal Perinatal Med. 2024. PMID: 38759030
-
Less invasive surfactant administration (LISA): chances and limitations.Arch Dis Child Fetal Neonatal Ed. 2019 Nov;104(6):F655-F659. doi: 10.1136/archdischild-2018-316557. Epub 2019 Jul 11. Arch Dis Child Fetal Neonatal Ed. 2019. PMID: 31296694 Free PMC article. Review.
-
Should less invasive surfactant administration (LISA) become routine practice in US neonatal units?Pediatr Res. 2023 Apr;93(5):1188-1198. doi: 10.1038/s41390-022-02265-8. Epub 2022 Aug 19. Pediatr Res. 2023. PMID: 35986148 Free PMC article. Review.
References
-
- Abman SH, Bancalari E, Jobe A. The evolution of bronchopulmonary dysplasia after 50 years. Am J Respir Crit Care Med. 2017;195:421–24. - PubMed
-
- Kribs A, Roll C, Göpel W, Wieg C, Groneck P, Laux R. NINSAPP Trial Investigators et al. Nonintubated surfactant application vs conventional therapy in extremely preterm infants: a randomized clinical trial. JAMA Pediatr. 2015;169:723–30. - PubMed
-
- Göpel W, Kribs A, Ziegler A, Laux R, Hoehn T, Wieg C. German Neonatal Network et al. Avoidance of mechanical ventilation by surfactant treatment of spontaneously breathing preterm infants (AMV): an open-label, randomised, controlled trial. Lancet. 2011;378:1627–34. - PubMed
-
- Kanmaz HG, Erdeve O, Canpolat FE, Mutlu B, Dilmen U. Surfactant administration via thin catheter during spontaneous breathing: randomized controlled trial. Pediatrics. 2013;131:e502–9. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

