Prefrontal parvalbumin interneurons mediate CRHR1-dependent early-life stress-induced cognitive deficits in adolescent male mice
- PMID: 39578519
- PMCID: PMC12092253
- DOI: 10.1038/s41380-024-02845-6
Prefrontal parvalbumin interneurons mediate CRHR1-dependent early-life stress-induced cognitive deficits in adolescent male mice
Abstract
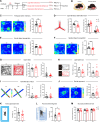
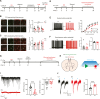
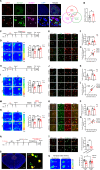
Cognitive impairment, a core symptom of psychiatric disorders, is frequently observed in adolescents exposed to early-life stress (ES). However, the underlying neural mechanisms are unclear, and therapeutic efficacy is limited. Targeting parvalbumin-expressing interneurons (PVIs) in the medial prefrontal cortex (mPFC), we report that ES reduces mPFC PVI activity, which causally mediated ES-induced cognitive deficits in adolescent male mice through chemogenetic and optogenetic experiments. To understand the possible causes of PVI activity reduction following ES, we then demonstrated that ES upregulated corticotropin-releasing hormone (CRH) receptor 1 [CRHR1, mainly expressed in pyramidal neurons (PNs)] and reduced activity of local pyramidal neurons (PNs) and their excitatory inputs to PVIs. The subsequent genetic manipulation experiments (CRHR1 knockout, CRH overexpression, and chemogenetics) highlight that ES-induced PVI activity reduction may result from CRHR1 upregulation and PN activity downregulation and that PVIs play indispensable roles in CRHR1- or PN-mediated cognitive deficits induced by ES. These results suggest that ES-induced cognitive deficits could be attributed to the prefrontal CRHR1-PN-PVI pathway. Finally, treatment with antalarmin (a CRHR1 antagonist) and environmental enrichment successfully restored the PVI activity and cognitive deficits induced by ES. These findings reveal the neurobiological mechanisms underlying ES-induced cognitive deficits in adolescent male mice and highlight the therapeutic potentials of PVIs in stress-related cognitive deficits in adolescent individuals.
© 2024. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests. Ethics approval and consent to participate: All methods performed in this study were conducted in accordance with the relevant guidelines and regulations. Ethical approval was obtained from the Peking University Biomedical Ethics Committee, Laboratory Animal Welfare Ethics Sub-Committee (reference number LA2020309). Informed consent was obtained from all participants involved in the study.
Figures



Similar articles
-
Dorsal CA1 NECTIN3 Reduction Mediates Early-Life Stress-Induced Object Recognition Memory Deficits in Adolescent Female Mice.Neurosci Bull. 2025 Feb;41(2):243-260. doi: 10.1007/s12264-024-01305-z. Epub 2024 Oct 12. Neurosci Bull. 2025. PMID: 39395912
-
Prefrontal Cortex Corticotropin-Releasing Factor Receptor 1 Conveys Acute Stress-Induced Executive Dysfunction.Biol Psychiatry. 2016 Nov 15;80(10):743-753. doi: 10.1016/j.biopsych.2016.03.2106. Epub 2016 Apr 8. Biol Psychiatry. 2016. PMID: 27318500
-
Deletion of the Mitochondrial Matrix Protein CyclophilinD Prevents Parvalbumin Interneuron Dysfunctionand Cognitive Deficits in a Mouse Model of NMDA Hypofunction.J Neurosci. 2020 Aug 5;40(32):6121-6132. doi: 10.1523/JNEUROSCI.0880-20.2020. Epub 2020 Jun 30. J Neurosci. 2020. PMID: 32605939 Free PMC article.
-
Corticotropin releasing hormone receptor 1 in the medial prefrontal cortex mediates aversion resistant alcohol intake.Psychopharmacology (Berl). 2024 Dec;241(12):2539-2550. doi: 10.1007/s00213-024-06707-5. Epub 2024 Oct 28. Psychopharmacology (Berl). 2024. PMID: 39466414
-
Forebrain CRHR1 deficiency attenuates chronic stress-induced cognitive deficits and dendritic remodeling.Neurobiol Dis. 2011 Jun;42(3):300-10. doi: 10.1016/j.nbd.2011.01.020. Epub 2011 Feb 3. Neurobiol Dis. 2011. PMID: 21296667 Free PMC article.
Cited by
-
Gut microbiota links to cognitive impairment in bipolar disorder via modulating synaptic plasticity.BMC Med. 2025 Aug 12;23(1):470. doi: 10.1186/s12916-025-04313-6. BMC Med. 2025. PMID: 40797316 Free PMC article.
-
Identification of blood transcriptome modules associated with suicidal ideation in patients with major depressive disorder.Sci Rep. 2025 Jan 7;15(1):1067. doi: 10.1038/s41598-025-85431-2. Sci Rep. 2025. PMID: 39774242 Free PMC article.
References
-
- Millan MJ, Agid Y, Brune M, Bullmore ET, Carter CS, Clayton NS, et al. Cognitive dysfunction in psychiatric disorders: characteristics, causes and the quest for improved therapy. Nat Rev Drug Discov. 2012;11:141–68. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

