Integrative metabolomics and proteomics reveal the effect and mechanism of Zi Qi decoction on alleviating liver fibrosis
- PMID: 39578538
- PMCID: PMC11584741
- DOI: 10.1038/s41598-024-80616-7
Integrative metabolomics and proteomics reveal the effect and mechanism of Zi Qi decoction on alleviating liver fibrosis
Abstract
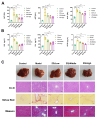
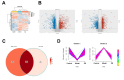
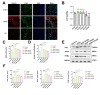
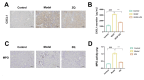
Liver fibrosis is a common progressive liver disease that can cause liver dysfunction and lead to serious complications. Zi Qi decoction (ZQ) is a traditional formulation that exerts pharmacological effects on the treatment of liver fibrosis. However, precise intervention mechanisms remain unclear. The aim of this study was to synergistically harness proteomics and metabolomics techniques to elucidate the specific target of ZQ and its potential mechanism of action. A carbon tetrachloride (CCl4)-induced liver fibrosis mouse model was established. Subsequently, the protective effect of ZQ on liver fibrosis mice was evaluated according to histopathological examination and biochemical indicators. Quantitative proteomics based on data independent acquisition (DIA) and non-targeted metabolomic analyses revealed the pharmacodynamic mechanism of ZQ. In addition, various cellular and molecular assays were used to detect changes in glycolysis levels in LSECs and mouse liver fibrosis models. The study results showed that ZQ significantly alleviated CCl4-induced liver injury and fibrosis in mice. DIA-based quantitative proteomics and non-targeted metabolomics analyses indicated that ZQ treatment downregulated glycolysis-related proteins such as PKM2, PFKP, and HK2, while regulating glycolysis-related metabolites and pathways. In addition, ZQ down-regulated glycolytic activity in mice with liver fibrosis and in LSECs, and inhibited CXCL1 secretion and neutrophil recruitment. ZQ inhibited LSEC glycolysis and mitigated neutrophil infiltration, thereby playing a therapeutic role in liver fibrosis.
Keywords: Glycolysis; Liver fibrosis; Liver sinusoidal endothelial cell; Neutrophil infiltration; Zi Qi Decoction.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests.
Figures




Similar articles
-
Quercetin alleviates liver fibrosis via regulating glycolysis of liver sinusoidal endothelial cells and neutrophil infiltration.Biomol Biomed. 2024 Oct 17;24(6):1806-1815. doi: 10.17305/bb.2024.10530. Biomol Biomed. 2024. PMID: 38943679 Free PMC article.
-
Xuefuzhuyu decoction inhibition of angiogenesis attenuates liver fibrosis induced by CCl₄ in mice.J Ethnopharmacol. 2014 May 14;153(3):659-66. doi: 10.1016/j.jep.2014.03.019. Epub 2014 Mar 15. J Ethnopharmacol. 2014. PMID: 24637190
-
Mechanotransduction-induced glycolysis epigenetically regulates a CXCL1-dominant angiocrine signaling program in liver sinusoidal endothelial cells in vitro and in vivo.J Hepatol. 2022 Sep;77(3):723-734. doi: 10.1016/j.jhep.2022.03.029. Epub 2022 Apr 12. J Hepatol. 2022. PMID: 35421427 Free PMC article.
-
Hepatoprotective effect of Xiayuxue decoction ethyl acetate fraction against carbon tetrachloride-induced liver fibrosis in mice via inducing apoptosis and suppressing activation of hepatic stellate cells.Pharm Biol. 2020 Dec;58(1):1229-1243. doi: 10.1080/13880209.2020.1855212. Pharm Biol. 2020. PMID: 33332219 Free PMC article.
-
Yinchenzhufu decoction ameliorates cholestatic liver injury through decreasing the formation of neutrophil extracellular traps in mice.J Ethnopharmacol. 2025 May 12;347:119720. doi: 10.1016/j.jep.2025.119720. Epub 2025 Mar 31. J Ethnopharmacol. 2025. PMID: 40174730
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

