STING induces HOIP-mediated synthesis of M1 ubiquitin chains to stimulate NF-κB signaling
- PMID: 39578541
- PMCID: PMC11696098
- DOI: 10.1038/s44318-024-00291-2
STING induces HOIP-mediated synthesis of M1 ubiquitin chains to stimulate NF-κB signaling
Abstract
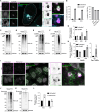
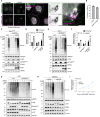
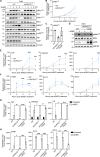
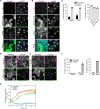
STING activation by cyclic dinucleotides induces IRF3- and NF-κB-mediated gene expression in mammals, as well as lipidation of LC3B at Golgi-related membranes. While mechanisms of the IRF3 response are well understood, the mechanisms of NF-κB activation via STING remain unclear. We report here that STING activation induces linear/M1-linked ubiquitin chain (M1-Ub) formation and recruitment of the LUBAC E3 ligase, HOIP, to LC3B-associated Golgi membranes where ubiquitin is also localized. Loss of HOIP prevents formation of M1-Ub chains and reduces STING-induced NF-κB and IRF3 signaling in human THP1 monocytes and mouse bone marrow-derived macrophages, without affecting STING activation. STING-induced LC3B lipidation is not required for M1-Ub chain formation or for immune-related gene expression, but the recently reported STING function in neutralizing Golgi pH may be involved. Thus, LUBAC synthesis of M1-linked ubiquitin chains mediates STING-induced innate immune signaling.
Keywords: Golgi; Innate Immunity; LC3B; LUBAC; NFkB.
© 2024. This is a U.S. Government work and not under copyright protection in the US; foreign copyright protection may apply.
Conflict of interest statement
Disclosure and competing interests statement. The authors declare no competing interests.
Figures





Update of
-
STING induces HOIP-mediated synthesis of M1 ubiquitin chains to stimulate NFκB signaling.bioRxiv [Preprint]. 2024 Oct 1:2023.10.14.562349. doi: 10.1101/2023.10.14.562349. bioRxiv. 2024. Update in: EMBO J. 2025 Jan;44(1):141-165. doi: 10.1038/s44318-024-00291-2. PMID: 37873486 Free PMC article. Updated. Preprint.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

