Predicting modular functions and neural coding of behavior from a synaptic wiring diagram
- PMID: 39578573
- PMCID: PMC11614741
- DOI: 10.1038/s41593-024-01784-3
Predicting modular functions and neural coding of behavior from a synaptic wiring diagram
Abstract
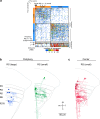
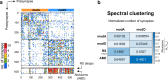
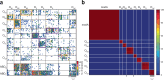
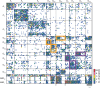
A long-standing goal in neuroscience is to understand how a circuit's form influences its function. Here, we reconstruct and analyze a synaptic wiring diagram of the larval zebrafish brainstem to predict key functional properties and validate them through comparison with physiological data. We identify modules of strongly connected neurons that turn out to be specialized for different behavioral functions, the control of eye and body movements. The eye movement module is further organized into two three-block cycles that support the positive feedback long hypothesized to underlie low-dimensional attractor dynamics in oculomotor control. We construct a neural network model based directly on the reconstructed wiring diagram that makes predictions for the cellular-resolution coding of eye position and neural dynamics. These predictions are verified statistically with calcium imaging-based neural activity recordings. This work demonstrates how connectome-based brain modeling can reveal previously unknown anatomical structure in a neural circuit and provide insights linking network form to function.
© 2024. The Author(s).
Conflict of interest statement
Competing interests: H.S.S., N.K., D.I., N.T. and K.L. have financial interests in Zetta AI, LLC. The remaining authors declare no competing interests.
Figures








References
-
- Briggman, K. L., Helmstaedter, M. & Denk, W. Wiring specificity in the direction-selectivity circuit of the retina. Nature471, 183–188 (2011). - PubMed
MeSH terms
Grants and funding
- CA203710/U.S. Department of Health & Human Services | NIH | NCI | Division of Cancer Epidemiology and Genetics, National Cancer Institute (National Cancer Institute Division of Cancer Epidemiology and Genetics)
- R01 EY021581/EY/NEI NIH HHS/United States
- NS104682/U.S. Department of Health & Human Services | NIH | National Institute of Neurological Disorders and Stroke (NINDS)
- R00 EY027017/EY/NEI NIH HHS/United States
- UH2 CA203710/CA/NCI NIH HHS/United States
- U19 NS104648/NS/NINDS NIH HHS/United States
- U19 NS132720/NS/NINDS NIH HHS/United States
- K99 EY027017/EY/NEI NIH HHS/United States
- EY021581/U.S. Department of Health & Human Services | NIH | National Eye Institute (NEI)
- EY027017/U.S. Department of Health & Human Services | NIH | National Eye Institute (NEI)
- EY027036/U.S. Department of Health & Human Services | NIH | National Eye Institute (NEI)
- R01 EY027036/EY/NEI NIH HHS/United States
- R01 NS104926/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources

